Abstract
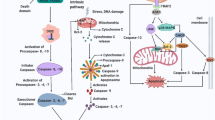
NALP3 inflammasome, which is an inflammatory caspase-activating complex, is composed of three proteins: NALP3 (an NOD-like receptor), an apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and caspase-1. NALP3 senses danger signals, while ASC is an adaptor molecule containing two protein interaction modules: pyrin domain (PYD) and caspase recruitment domain (CARD). Caspase-1 is a cysteine protease that uses cysteine as a nucleophile and has a CARD domain for protein interaction. During inflammasome formation, the ASC adaptor acts as a bridge between caspase and NOD-like receptor (NLR) by offering the CARD for CARD–CARD interactions and PYD for PYD–PYD interactions. In the current study, we successfully purified and characterized NALP3 PYD and ASC PYD. The results showed that ASC PYD easily self-oligomerized under physiological conditions, and this self-oligomerization of the ASC PYD prevented complex formation with NALP3 PYD in vitro.






Similar content being viewed by others
References
Ghayur, T., Banerjee, S., Hugunin, M., Butler, D., Herzog, L., Carter, A., Quintal, L., Sekut, L., Talanian, R., Paskind, M., Wong, W., Kamen, R., Tracey, D., & Allen, H. (1997). Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature, 386, 619–623.
Kanneganti, T. D., Ozoren, N., Body-Malapel, M., Amer, A., Park, J. H., Franchi, L., Whitfield, J., Barchet, W., Colonna, M., Vandenabeele, P., Bertin, J., Coyle, A., Grant, E. P., Akira, S., & Nunez, G. (2006). Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature, 440, 233–236.
Sutterwala, F. S., Ogura, Y., Szczepanik, M., Lara-Tejero, M., Lichtenberger, G. S., Grant, E. P., Bertin, J., Coyle, A. J., Galan, J. E., Askenase, P. W., & Flavell, R. A. (2006). Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity, 24, 317–327.
Dostert, C., Petrilli, V., Van Bruggen, R., Steele, C., Mossman, B. T., & Tschopp, J. (2008). Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science, 320, 674–677.
Martinon, F., Burns, K., & Tschopp, J. (2002). The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular Cell, 10, 417–426.
Davis, B. K., Wen, H., & Ting, J. P. (2011). The inflammasome NLRs in immunity, inflammation, and associated diseases. Annual Review of Immunology, 29, 707–735.
Agostini, L., Martinon, F., Burns, K., McDermott, M. F., Hawkins, P. N., & Tschopp, J. (2004). NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity, 20, 319–325.
Schroder, K., & Tschopp, J. (2010). The inflammasomes. Cell, 140, 821–832.
Bae, J. Y., & Park, H. H. (2011). Crystal structure of NALP3 protein pyrin domain (PYD) and its implications in inflammasome assembly. Journal of Biological Chemistry, 286, 39528–39536.
Martinon, F. (2008). Detection of immune danger signals by NALP3. Journal of Leukocyte Biology, 83, 507–511.
Duncan, J. A., Bergstralh, D. T., Wang, Y., Willingham, S. B., Ye, Z., Zimmermann, A. G., & Ting, J. P. (2007). Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proceedings of the National Academy of Sciences of the United States of America, 104, 8041–8046.
Shiohara, M., Taniguchi, S., Masumoto, J., Yasui, K., Koike, K., Komiyama, A., & Sagara, J. (2002). ASC, which is composed of a PYD and a CARD, is up-regulated by inflammation and apoptosis in human neutrophils. Biochemical and Biophysical Research Communications, 293, 1314–1318.
Srimathi, T., Robbins, S. L., Dubas, R. L., Chang, H., Cheng, H., Roder, H., & Park, Y. C. (2008). Mapping of POP1-binding site on pyrin domain of ASC. Journal of Biological Chemistry, 283, 15390–15398.
Park, H. H. (2012). PYRIN domains and their interactions in the apoptosis and inflammation signaling pathway. Apoptosis, 17, 1247–1257.
Franchi, L., Eigenbrod, T., Munoz-Planillo, R., & Nunez, G. (2009). The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nature Immunology, 10, 241–247.
Jang, T. H., & Park, H. H. (2011). Generalized semi-refolding methods for purification of the functional death domain superfamily. Journal of Biotechnology, 151, 335–342.
Park, H. H. (2011). Structural analyses of death domains and their interactions. Apoptosis, 16, 209–220.
Dzivenu, O. K., Park, H. H., & Wu, H. (2004). General co-expression vectors for the overexpression of heterodimeric protein complexes in Escherichia coli. Protein Expression and Purification, 38, 1–8.
Chen, Y. H., Yang, J. T., & Martinez, H. M. (1972). Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry, 11, 4120–4131.
Jang, T. H., Bae, J. Y., Park, O. K., Kim, J. H., Cho, K. H., Jeon, J. H., & Park, H. H. (2010). Identification and analysis of dominant negative mutants of RAIDD and PIDD. Biochimica et Biophysica Acta, 1804, 1557–1563.
Park, H. H., Logette, E., Rauser, S., Cuenin, S., Walz, T., Tschopp, J., & Wu, H. (2007). Death domain assembly mechanism revealed by crystal structure of the oligomeric PIDDosome core complex. Cell, 128, 533–546.
Wang, L., Yang, J. K., Kabaleeswaran, V., Rice, A. J., Cruz, A. C., Park, A. Y., Yin, Q., Damko, E., Jang, S. B., Raunser, S., Robinson, C. V., Siegel, R. M., Walz, T., & Wu, H. (2010). The Fas-FADD death domain complex structure reveals the basis of DISC assembly and disease mutations. Nature Structural and Molecular Biology, 17, 1324–1329.
Lin, S. C., Lo, Y. C., & Wu, H. (2010). Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature, 465, 885–890.
Qin, H., Srinivasula, S. M., Wu, G., Fernandes-Alnemri, T., Alnemri, E. S., & Shi, Y. (1999). Structural basis of procaspase-9 recruitment by the apoptotic protease-activating factor 1. Nature, 399, 549–557.
Kwon, D., Yoon, J. H., Shin, S. Y., Jang, T. H., Kim, H. G., So, I., Jeon, J. H., & Park, H. H. (2011). A comprehensive manually curated protein-protein interaction database for the Death Domain superfamily. Nucleic Acids Research, 40, D331–D336.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) of the Ministry of Education, Science and Technology (2013009083) and a grant from the Korea Healthcare Technology R&D project, Ministry of Health & Welfare, Republic of Korea (HI13C1449).
Author information
Authors and Affiliations
Corresponding author
Additional information
Kannan Badri Narayanan and Tae-Ho Jang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Narayanan, K.B., Jang, TH. & Park, H.H. Self-oligomerization of ASC PYD Domain Prevents the Assembly of Inflammasome In Vitro. Appl Biochem Biotechnol 172, 3902–3912 (2014). https://doi.org/10.1007/s12010-014-0819-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-0819-0