Abstract
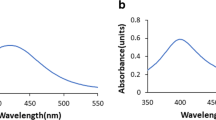
The antifungal activity of polyvinylpyrrolidone (PVP)-stabilized quantum-sized silver nanoparticles (SNPs) against the growth of Candida albicans has been demonstrated in the present study. C. albicans is a known opportunistic human pathogen causing superficial and systemic infections. Research data carried out on C. albicans so far have shown unequivocally that it develops resistance against conventional antifungal drugs and that the infections it causes are difficult to cure with conventional antifungal agents. Hence, it is urgent to find newer materials for the treatment of infections caused by C. albicans that must be safe for the host. PVP-capped SNPs were synthesized, and its surface plasmon band was observed at 410 nm. The growth of C. albicans was markedly inhibited when the cells were incubated with SNP. The minimum inhibitory concentration (MIC) of SNP was determined as 70 ng/ml, and this value is relatively lower when compared with the conventionally used antifungal drugs such as amphotericin B (0.5 μg/ml), fluconazole (0.5 μg/ml), and ketoconazole (8 μg/ml). The viability of SNP-treated cells was checked by measuring the metabolic activity using XTT assay. Field emission scanning electron microscopic (FE-SEM) and transmission electron microscopic (TEM) analyses of the cells treated with SNP have lost the structural integrity to a greater extent.






Similar content being viewed by others
References
Perlroth, J., Choi, B., & Spellberg, B. (2007). Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Medical Mycology, 45, 321–346.
Goffeau, A. (2008). Drug resistance: the fight against fungi. Nature, 452, 541–542.
Jin, Y., Samaranayake, L. P., Samaranayake, Y., & Yip, H. K. (2004). Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Archives of Oral Biology, 49(10), 789–798.
Levin, M. D., Hollander, J. G. D., Van der Holt, B., Rijnders, B. J., Vliet, M. V., Sonneveld, P., et al. (2007). Hepatotoxicity of oral and intravenous voriconazole in relation to cytochrome P450 polymorphisms. Journal of Antimicrobial Chemotherapy, 60(5), 1104–1107.
Taxvig, C., Hass, U., Axelstad, M., Dalgaard, M., Boberq, J., Andeasen, H. R., et al. (2007). Endocrine-disrupting activities in vivo of the fungicides tebuconazole and epoxiconazole. Toxicological Sciences, 100(2), 464–473.
Worth, L. J., Blyth, C. C., Booth, D. L., Kong, D. C., Marriott, D., Cassumbhoy, M., et al. (2008). Optimising antifungal drug dosing and monitoring to avoid toxicity and improve outcomes in patients with haematological disorders. Internal Medicine Journal, 38(6b), 521–537.
Venkatakrishnan, K., Von Moltke, L. L., & Greenblatt, D. J. (2000). Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clinical Pharmacokinetics, 38(2), 111–180.
White, T. C., Holleman, S., Dy, F., Mirels, L. F., & Stevens, D. A. (2002). Resistance mechanisms in clinical isolates of Candida albicans. Antimicrobial Agents and Chemotherapy, 46(6), 1704–1713.
Perea, S., Lopez-Ribot, J. L., Kirkpatrick, W. R., McAtee, R. K., Santillan, R. A., Martinez, M., et al. (2001). Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrobial Agents and Chemotherapy, 45(10), 2676–2684.
Silver, S. (2003). Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiology Reviews, 27, 341–353.
Klasen, H. J. (2000). A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns, 26(2), 131–138.
Baker, C., Pradhan, A., Pakstis, L., Pochan, D. J., & Shah, S. I. (2005). Synthesis and antimicrobial properties of silver nanoparticles. Journal of Nanoscience and Nanotechnology, 5(2), 244–249.
Melaiye, A., Sun, Z., Hindi, K., Milsted, A., Ely, D., Reneker, D. H., et al. (2005). Silver(I)-imidazole cyclophane gem-diol complexes encapsulated by electrospun tecophilic nanofibers: formation of nanosilver particles and antimicrobial activity. Journal of the American Chemical Society, 127(7), 2285–2291.
Sondi, I., & Salopek-Sondi, B. (2004). Silver nanoparticles as antimicrobialagent: a case study on E. coli as a model for Gram-negative bacteria. Journal of Colloid and Interface Science, 275(1), 177–182.
Liversidge, E. M., Liversidge, G. G., & Copper, E. R. (2003). Nanosizing: A formulation approach for poorly- water- soluble compounds. European Journal of Pharmaceutical Sciences, 18(2), 113–120.
Brigger, I., Dubernet, C., & Couvreur, P. (2002). Nanoparticles in cancer therapy and diagnosis. Advanced Drug Delivery Reviews, 54, 631–652.
CLSI (2008b). Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard CLSI document M38-A2. Wayne: Clinical and Laboratory Standards Institute.
Hartsel, S., & Bolard, J. (1996). Amphotericin B: new life for an old drug. Trends in Pharmacological Sciences, 17, 445–449.
Ramage, G., Vande Walle, K., Wickes, B. L., & Lopez-Rebot, J. L. (2001). Standardisation method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrobial Agents and Chemotheraphy, 45, 2475–2479.
Chan, W. C. W., Maxwell, D. J., Gao, X. H., Bailey, R. E., Han, M. Y., & Nie, S. M. (2002). Luminescent quantum dots for multiplexed biological detection and imaging. Current Opinion Biotechnology, 13(1), 40–46.
Sondi, I., Siiman, O., Koester, S., & Matijevic, E. (2000). Preparation of aminodextran- CdS nanoparticle complexes and biologically active antibody-amino dextran-CdS nanoparticle conjugates. Langmuir, 16, 3107–3118.
Zhao, G. J., & Stevens, S. E. (1998). Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. Biometals, 11, 27–32.
Herrera, M., Carrion, P., Baca, P., Liebana, J., & Castillo, A. (2001). In vitro antibacterial activity of glass-ionomer cements. Microbios, 104, 141–148.
Klaus, T., Joerger, R., Olsson, E., & Granqvist, C. G. (1999). Silver-based crystalline nanoparticles, microbially fabricated. Proceedings of National Academy of Sciences, USA, 96, 13611–13614.
Wu, C., Mosher, B. P., Lyons, K., & Zeng, T. (2010). Reducing ability and mechanism for Polyvinylpyrrolidone (PVP) in silver nanoparticles synthesis. Journal of Nanoscience and Nanotechnology, 10, 2342–2347.
Sahoo, P. K., Kalyan Kamal, S. S., Jagadeesh Kumar, T., Sreedhar, B., Singh, A. K., & Srivastava, S. K. (2009). Synthesis of silver nanoparticles using facile wet chemical route. Defence Science Journal, 59(4), 447–455.
Jiang, L. P., Wang, A. N., Zhao, Y., Zhang, J. R., & Zhu, J. J. (2004). A novel route for the preparation of monodisperse silvernanoparticles via a pulsed sonoelectrochemical technique. Inorganic Chemistry Communications, 7(4), 506–509.
Kvitek, L., Panacek, A., Soukupova, J., Kol, M., Vecero, R., Prucek, R., et al. (2008). Effect of surfactant and polymers on stability and antibacterial activity of silver Nanoparticles (NPs). Journal of Physical Chemistry C, 112, 5825–5834.
Kim, K. J., Sung, W. S., Suh, B. K., Moon, S. K., Choi, J. S., Kim, J. G., et al. (2009). Antifungal activity and mode of action of silver nano-particles on Candida albicans. Biometals, 22, 235–242.
Hwang, E. T., Lee, J. H., Chae, Y. J., Kim, Y. S., Kim, B. C., Sang, B. J., et al. (2008). Analysis of the toxic mode of action of silver nanoparticles using stress-specific bioluminescent bacteria. Small, 4, 746–750.
Niazi, J. H., Sang, B. I., Kim, Y. S., & Gu, M. B. (2011). Global Gene Response in Saccharomyces cerevisiae exposed to silver nanoparticles. Applied Biochemistry and Biotechnology, 164(8), 1278–1291.
Klis, F. M., de Groot, P., & Hellingwerf, K. (2001). Molecular organization of the cell wall of Candida albicans. Medical Mycology, 39(1), 1–8.
Latgé, J. P. (2010). Tasting the fungal cell wall. Cellular Microbiology, 12(7), 863–872.
Cope, J. E. (1980). The porosity of the cell wall of Candida albicans. Journal of General Microbiology, 119, 253–255.
Basrai, M. A., Naider, F., & Becker, J. M. (1990). Internalization of lucifer yellow in Candida albicans by fluid phase endocytosis. Journal of General Microbiology, 136(6), 1059–1065.
Etxeberria, E., Gonzalez, P., Fernandez, E. B., & Romero, J. P. (2006). Fluid phase endocytic uptake of artificial nano-spheres and fluorescent quantum dots by sycamore cultured cells. Plant Signaling and Behavior, 1(4), 196–200.
Johnston, D. A., Eberle, K. E., Sturtevant, J. E., & Palmer, G. E. (2009). Role for endosomal and vacuolar GTPases in Candida albicans pathogenesis. Infection and Immunity, 77(6), 2343–2355.
Berman, J. (2006). Morphogenesis and cell cycle progression in Candida albicans. Current Opinion in Microbiology, 9, 595–601.
Hwang, I. S., Lee, J., Hwang, J. H., Kim, K. J., & Lee, D. G. (2012). Silver nanoparticles induce apoptotic cell death in Candida albicans through the increase of hydroxyl radicals. FEBS Journal, 279(7), 1327–1338.
Elechiguerra, J. L., Burt, J. L., Morones, J. R., Bragado, A. C., Gao, X., Lara, H. H., et al. (2005). Interaction of silver nanoparticles with HIV-1. Journal of Nanobiotechnology, 3, 6.
Sun, L., Singh, A. K., Vig, K., Pillai, S. R., & Singh, S. R. (2008). Silver nanoparticles inhibit replication of respiratory syncytial virus. Journal of Biomedical Nanotechnology, 4, 149–158.
Morones, J. R., Elechiguerra, J. L., Camacho, A., Holt, K., Kouri, J. B., Ramirez, J. T., et al. (2005). The bactericidal effect of silver nanoparticles. Journal of Nanotechnology, 16(10), 2346–2353.
Kim, S. H., Lee, H. S., Ryu, D. S., Choi, S. J., & Lee, D. S. (2011). Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean Journal of Microbiology and Biotechnology, 39(1), 77–85.
Pal, S., Tak, Y. K., & Song, J. M. (2007). Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Applied and Environmental Microbiology, 73(6), 1712–1720.
Panacek, A., Kolar, M., Vecerova, R., Prucek, R., Soukupova, J., Krystof, V., et al. (2009). Antifungal activity of silver nanoparticles against Candida spp. Biomaterials, 30(31), 6333–6340.
Kvitek, L., Vanickova, M., Panacek, A., Soukupova, J., Dittrich, M., Valentova, E., et al. (2009). Initial study on the toxicity of silver nanoparticles (NPs) against Paramecium caudatum. Journal of Physical Chemistry C, 113, 4296–4300.
Lee, H. J., Lee, S. G., Oh, E. J., Chung, H. Y., Han, S. I., Kim, E. J., et al. (2011). Antimicrobial polyethyleneimine silver nanoparticles in a stable colloidal dispersion. Colloids and Surfaces B: Biointerfaces, 88(1), 505–511.
Choi, O., & Hu, Z. (2008). Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environmental Science Technology, 42, 4583–4588.
Acknowledgments
The authors thank the National Centre for Nanoscience and Nanotechnology, MHRD and DST-INSPIRE for financial support in the form of a research grant and junior research fellowships. Thanks is also due to Mr S. Prathap Augustine, technician, NCNSNT, for assisting us with FE-SEM and TEM imaging.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Selvaraj, M., Pandurangan, P., Ramasami, N. et al. Highly Potential Antifungal Activity of Quantum-Sized Silver Nanoparticles Against Candida albicans . Appl Biochem Biotechnol 173, 55–66 (2014). https://doi.org/10.1007/s12010-014-0782-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-0782-9