Abstract
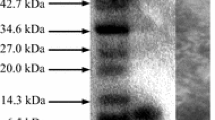
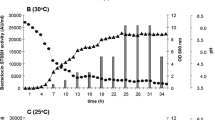
Bacteriocins are low molecular peptides with antimicrobial activity, which are of great interest as food bio-preservatives and for treating diseases caused by pathogenic bacteria. In this study, we present the characterization of bacteriocins produced by Lactobacillus plantarum LE5 and LE27 isolated from ensiled corn. Bacteriocins were purified through ammonium sulfate precipitation and double dialysis by using 12- and 1-kDa membranes. Bacteriocins showed activity against Listeria innocua, Listeria monocytogenes, and Enteroccocus faecalis. Molecular weight was estimated through Tricine-SDS-PAGE and overloading the gel onto Mueller-Hinton agar seeded with L. monocytogenes, showing an inhibition zone between 5 and 10 kDa. NanoLC-MS/MS analysis allowed the identification of UPF0291 protein (UniProtKB/Swiss-Prot Q88VI7), which is also presented in other lactic acid bacteria without assigned function. Ab initio modeling showed it has an α-helix-rich structure and a large positive-charged region. Bacteriocins were stable between 4 and 121 °C and pH 2 and 12, and the activity was inhibited by SDS and proteases. Mode of action assay suggests that the bacteriocin causes of target microorganism. Taken together, these results describe a possible new class IIa bacteriocin produced by L. plantarum, which has a wide stability to physicochemical conditions, and that could be used as an alternative for the control of foodborne diseases.









Similar content being viewed by others
References
Rajilic-Stojanovic, M., Smidt, H., & de Vos, W. M. (2007). Diversity of the human gastrointestinal tract microbiota revisited. Environmental Microbiology, 9, 2125–2136.
Wallace, T. C., Guarner, F., Madsen, K., Cabana, M. D., Gibson, G., Hentges, E., et al. (2011). Human gut microbiota and its relationship to health and disease. Nutrition Reviews, 69, 392–403.
Dicks, L. M. T., & Botes, M. (2010). Probiotic lactic acid bacteria in the gastro-intestinal tract: health benefits, safety and mode of action. Benef Microbes, 1, 11–29.
Masood, M. I., Qadir, M. I., Shirazi, J. H., & Khan, I. U. (2011). Beneficial effects of lactic acid bacteria on human beings. Critical Reviews in Microbiology, 37, 91–98.
Badel, S., Bernardi, T., & Michaud, P. (2011). New perspectives for lactobacilli exopolysaccharides. Biotechnology Advances, 29, 54–66.
Servin, A. L. (2004). Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiology Reviews, 28, 405–440.
Benmechernene, Z., Fernandez-No, I., Kihal, M., Böhme, K., Calo-Mata, P., & Barros-Velazquez, J. (2013). Recent patents on bacteriocins: food and biomedical applications. Recent Patents on DNA & Gene Sequences, 7, 66–73.
Hammami, R., Fernandez, B., Lacroix, C., Fliss, I. (2012). Anti-infective properties of bacteriocins: an update. Cell. Mol. Life Sci.
Cotter, P. D., Ross, R. P., & Hill, C. (2013). Bacteriocins—a viable alternative to antibiotics? Nature Reviews Microbiology, 11, 95–105.
Cotter, P. D., Hill, C., & Ross, R. P. (2005). Bacteriocins: developing innate immunity for food. Nature Reviews Microbiology, 3, 777–788.
Drider, D., Fimland, G., Héchard, Y., McMullen, L. M., & Prévost, H. (2006). The continuing story of class IIa bacteriocins. Microbiology and Molecular Biology Reviews, 70, 564–582.
Papagianni, M., & Papamichael, E. M. (2011). Purification, amino acid sequence and characterization of the class IIa bacteriocin weissellin A, produced by Weissella paramesenteroides DX. Bioresource Technology, 102, 6730–6734.
Todorov, S. D., & Dicks, L. M. (2009). Bacteriocin production by Pediococcus pentosaceus isolated from marula (Scerocarya birrea). International Journal of Food Microbiology, 132, 117–126.
Maldonado-Barragan, A., Caballero-Guerrero, B., Jimenez, E., Jimenez-Diaz, R., Ruiz-Barba, J. L., & Rodriguez, J. M. (2009). Enterocin C, a class IIb bacteriocin produced by E. faecalis C901, a strain isolated from human colostrum. International Journal of Food Microbiology, 133, 105–112.
Todorov, S. D., Rachman, C., Fourrier, A., Dicks, L. M. T., van Reenen, C. A., Prévost, H., et al. (2011). Characterization of a bacteriocin produced by Lactobacillus sakei R1333 isolated from smoked salmon. Anaerobe, 17, 23–31.
Hata, T., Tanaka, R., & Ohmomo, S. (2010). Isolation and characterization of plantaricin ASM1: a new bacteriocin produced by Lactobacillus plantarum A-1. International Journal of Food Microbiology, 137, 94–99.
Muñoz, M., Mosquera, A., Alméciga-Díaz, C. J., Melendez, A. P., & Sánchez, O. F. (2012). Fructooligosaccharides metabolism and effect on bacteriocin production in Lactobacillus strains isolated from ensiled corn and molasses. Anaerobe, 18, 321–330.
Todorov, S. D., Ho, P., Vaz-Velho, M., & Dicks, L. M. (2010). Characterization of bacteriocins produced by two strains of Lactobacillus plantarum isolated from Beloura and Chouriço, traditional pork products from Portugal. Meat Science, 84, 334–343.
Schägger, H., & von Jagow, G. (1987). Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Analytical Biochemistry, 166, 368–379.
Campos, C. A., Rodríguez, Ó., Calo-Mata, P., Prado, M., & Barros-Velázquez, J. (2006). Preliminary characterization of bacteriocins from Lactococcus lactis, Enterococcus faecium and Enterococcus mundtii strains isolated from turbot (Psetta maxima). Food Research International, 39, 356–364.
Chevallet, M., Luche, S., & Rabilloud, T. (2006). Silver staining of proteins in polyacrylamide gels. Nature Protocols, 1, 1852–1858.
Xu, D., & Zhang, Y. (2012). Ab initio protein structure assembly using continuous structure fragments and optimized knowledge-based force field. Proteins, 80, 1715–1735.
Laskowski, R. A. (2009). PDBsum new things. Nucleic Acids Research, 37, D355–D359.
Martinez, R. C., Wachsman, M., Torres, N. I., LeBlanc, J. G., Todorov, S. D., & Franco, B. D. (2013). Biochemical, antimicrobial and molecular characterization of a noncytotoxic bacteriocin produced by Lactobacillus plantarum ST71KS. Food Microbiology, 34, 376–381.
Nowroozi, J., Mirzaii, M., & Norouzi, M. (2004). Study of Lactobacillus as probiotic bacteria. Iranian Journal of Public Health, 33, 1–7.
Cintas, L., Herranz, C., Hernández, P., Casaus, M., & Nes, L. (2001). Review: bacteriocins of lactic acid bacteria. Food Science and Technology International, 7, 281–305.
Todorov, S. D., & Dicks, L. M. (2005). Characterization of bacteriocins produced by lactic acid bacteria isolated from spoiled black olives. Journal of Basic Microbiology, 45, 312–322.
Todorov, S. D., & Dicks, L. M. T. (2006). Screening for bacteriocin-producing lactic acid bacteria from boza, a traditional cereal beverage from Bulgaria: comparison of the bacteriocins. Process Biochemistry, 46, 11–19.
Hammami, R., Zouhir, A., Le Lay, C., Ben Hamida, J., & Fliss, I. (2010). BACTIBASE second release: a database and tool platform for bacteriocin characterization. BMC Microbiology, 10, 22.
Todorov, S. D., van Reenen, C. A., & Dicks, L. M. (2004). Optimization of bacteriocin production by Lactobacillus plantarum ST13BR, a strain isolated from barley beer. Journal of General and Applied Microbiology, 50, 149–157.
Zouhir, A., Hammami, R., Fliss, I., & Hamida, J. B. (2010). A new structure-based classification of gram-positive bacteriocins. Protein Journal, 29, 432–439.
Nes, I. F., & Johnsborg, O. (2004). Exploration of antimicrobial potential in LAB by genomics. Current Opinion in Biotechnology, 15, 100–104.
Gálvez, A., Abriouel, H., López, R. L., & Ben Omar, N. (2007). Bacteriocin-based strategies for food biopreservation. International Journal of Food Microbiology, 120, 51–70.
Todorov, S. D., & Dicks, L. M. T. (2005). Lactobacillus plantarum isolated from molasses produces bacteriocins active against Gram-negative bacteria. Enzyme and Microbial Technology, 36, 318–326.
Zapata, S., Muñoz, J., Ruiz, O., Montoya, O., & Gutierrez, P. (2009). Isolation of Lactobacillus plantarum LPBM10 and partial characterization of its bacteriocin. Vitae, 16, 75–82.
Mourad, K., Halima, Z.-K., & Nour-Eddine, K. (2005). Detection and activity of plantaricin OL15 a bacteriocin produced by Lactobacillus plantarum OL15 isolated from Algerian fermented olives. Grasas y Aceites, 56, 192–197.
Sowani, H. M., & Thorat, P. (2012). Antimicrobial activity studies of bactoriocin produced by Lactobacilli isolates from carrot kanji. OnLine Journal of Biological Sciences, 12, 6–10.
Lee, N. K., & Paik, H. D. (2001). Partial characterisation of lactocin NK24, a newly identified bacteriocin of Lactococcus lactis NK24 isolated from Jeot-gal. Food Microbiology, 18, 17–24.
Ferchichi, M., Frère, J., Mabrouk, K., & Manai, M. (2001). Lactococcin MMFII, a novel class IIa bacteriocin produced by Lactococcus lactis MMFII, isolated from a Tunisian dairy product. FEMS Microbiology Letters, 205, 49–55.
Todorov, S., & Dicks, L. M. T. (2005). Pediocin ST18, an anti-listerial bacteriocin produced by Pediococcus pentosaceus ST18 isolated from boza, a traditional cereal beverage from Bulgaria. Process Biochemistry, 40, 365–370.
Huot, E., Meghrous, J., Barrena-Gonzalez, C., & Petitdemange, H. (1996). Bacteriocin J46, a new bacteriocin produced by Lactococcus lactis subsp. cremoris J46: isolation and characterization of the protein and its gene. Anaerobe, 2, 137–145.
Nissen-Meyer, J., Rogne, P., Oppegård, C., Haugen, H. S., & Kristiansen, P. E. (2009). Structure-function relationships of the non-lanthionine-containing peptide (class II) bacteriocins produced by gram-positive bacteria. Current Opinion in Biotechnology, 10, 19–37.
Gómez, N. C., Abriouel, H., Grande, M. A., Pulido, R. P., & Gálvez, A. (2012). Effect of enterocin AS-48 in combination with biocides on planktonic and sessile Listeria monocytogenes. Food Microbiology, 30, 51–58.
Brandt, A. L., Castillo, A., Harris, K. B., Keeton, J. T., Hardin, M. D., & Taylor, T. M. (2010). Inhibition of Listeria monocytogenes by food antimicrobials applied singly and in combination. Journal of Food Science, 75, M557–M563.
Neetoo, H., Ye, M., & Chen, H. (2008). Potential antimicrobials to control Listeria monocytogenes in vacuum-packaged cold-smoked salmon pâté and fillets. International Journal of Food Microbiology, 123, 220–227.
Shelburne, C. E., An, F. Y., Dholpe, V., Ramamoorthy, A., Lopatin, D. E., & Lantz, M. S. (2007). The spectrum of antimicrobial activity of the bacteriocin subtilosin A. Journal of Antimicrobial Chemotherapy, 59, 297–300.
Mills, S., Serrano, L. M., Griffin, C., O'Connor, P. M., Schaad, G., Bruining, C., et al. (2011). Inhibitory activity of Lactobacillus plantarum LMG P-26358 against Listeria innocua when used as an adjunct starter in the manufacture of cheese. Microbial Cell Factories, 10(Suppl 1), S7.
Aguilar, C., Vanegas, C., Klotz, B. (2010). Antagonistic effect of Lactobacillus strains against Escherichia coli and Listeria monocytogenes in milk. The Journal of Dairy Research, 3, 1–8.
Gong, H. S., Meng, X. C., & Wang, H. (2010). Mode of action of plantaricin MG, a bacteriocin active against Salmonella typhimurium. Journal of Basic Microbiology, 50(Suppl 1), S37–S45.
Acknowledgments
This project was supported by Universidad Nacional de Colombia (project no. 14729) and Pontificia Universidad Javeriana (ID 004347). We thank Dr. Ricardo Vera and Max Martinez from Laboratorio de Macromoléculas and to the Unidad de Investigaciones Agropecuarias at Pontificia Universidad Javeriana.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Amortegui, J., Rodríguez-López, A., Rodríguez, D. et al. Characterization of a New Bacteriocin from Lactobacillus plantarum LE5 and LE27 Isolated from Ensiled Corn. Appl Biochem Biotechnol 172, 3374–3389 (2014). https://doi.org/10.1007/s12010-014-0757-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-0757-x