Abstract
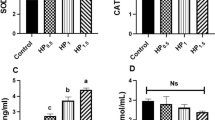
Seawater bittern (nigari) is a concentrated solution remaining after the crystallization process of salt that has been used as a coagulant for tofu. Recently, various nigari products are distributed in the East Asia. To clarify the properties of nigari products, major mineral composition of six nigari products was determined. Then, effects of the nigari on the browning and antioxidant activity during the glucose/lysine Maillard reaction were investigated. Though the predominant cation was Mg2+, the content was varied by each product (0.88–6.49 mol/L). The other major ion contents were also varied. Each 0.5 mol/L of d-glucose and l-lysine were incubated with the nigari (5–50 % (v/v)) or nigari-related salts (1 or 2 mol/L). The browning (OD at 465 nm) and antioxidant activity (1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging capacity and ferrous-reducing power) were increased remarkably by the nigari containing high Mg2+ content. The browning tended to be high with sulfates (Na2SO4, (NH4)2SO4). On the other hand, high content of MgCl2 decreased slightly the browning and antioxidant activity. These results suggest that the reaction and antioxidant activities were affected not only by salinity and cations but also by anions and other elements in the nigari.







Similar content being viewed by others
References
Ayoub, G. M., Merhebi, F., Acra, A., El-Fadei, M., & Koopman, B. (2000). Water Research, 34, 640–656.
Lychnos, G., Fletcher, J. P., & Davies, P. A. (2010). Desalination, 250, 172–178.
Tsai, S. J., Lan, C. Y., Kao, C. S., & Chen, S. C. (1981). Studies on the yield and quality characteristics of tofu. Journal of Food Science, 46, 1734–1737.
Haga, M., Niino, Y., Nishimura, H., & Seki, Y. (2005). Journal of Cookery Science of Japan, 38, 281–285.
Yokota, K., Kato, M., Lister, F., Ii, H., Hayakawa, T., Kikuta, T., Kageyama, S., & Tajima, N. (2004). Journal of the American College of Nutrition, 23, 506S–509S.
Venskutonis, R. P., Vasillauskalit, R., Galdikas, A., & Setkus, S. (2002). Food Control, 13, 13–21.
Wang, H. Y., Qian, H., & Yao, W. R. (2011). Food Chemistry, 128, 573–584.
Kuda, T., Hishi, T., & Maekawa, S. (2006). Food Chemistry, 98, 545–550.
Kuda, T., & Yano, T. (2009). LWT--Food Science and Technology, 42, 1070–1075.
Tuohy, K. M., Hinton, D. J. S., Davis, S. J., Crabbe, M. J. C., Gibson, G. R., & Ames, J. M. (2006). Molecular Nutrition & Food Research, 50, 847–857.
Kataoka, S. (2005). Journal of Bioscience and Bioengineering, 100, 227–234.
Kwak, E. J., & Lim, S. I. (2004). Amino Acid, 27, 85–90.
Matiacevich, S. M., Santagapita, P. R., & del Pilar-Buera, M. (2010). Food Chemistry, 118, 103–108.
Dwyer, J., Strrenburg, D., Tait, S., Barr, K., Batstone, D. J., & Lant, P. (2008). Water Research, 42, 4699–4709.
Yan, P. (2011). Experimental Gerontology, 46, 847–852.
Schöneich, C. (2005). BBA-Proteins and Proteomics, 1703, 111–119.
Fukai, M. U., & Nakamura, Y. (2008). Cancer Letter, 266, 37–52.
Kuda, T., Kunii, T., Goto, H., Suzuki, T., & Yano, T. (2007). Food Chemistry, 103, 900–905.
Kuda, T., & Ikemori, T. (2009). Food Chemistry, 112, 575–581.
Blois, M. S. (1958). Nature, 26, 1199–1200.
Zhu, Q. T., Hackman, R., Ensunsa, J. L., Holt, R. R., & Keen, C. L. (2002). Journal of Agricultural and Food Chemistry, 50, 6929–6934.
Gokmen, V., & Senyuva, H. (2007). Food Chemistry, 103, 196–203.
Ogimoto, M., Uematsu, Y., Kabashima, J., Suzuki, K., & Ito, K. (2006). Shokuhin Eiseigaku Zasshi, 47, 296–301.
Kanno, T., Kuda, T., An, C., Takahashi, H., & Kimura, B. (2012). LWT--Food Science and Technology, 47, 25–30.
Alves-Silva, J. M., dos Santos, S. M., Pintado, M. E., Perez-Alverez, J. A., Fernandez-Lopez, J., & Viuda-Martos, M. (2013). Food Control, 32, 371–378.
Kuda, T., Nakamura, S., An, C., Takahashi, H., Kimura, B., & Nishizawa, M. (2012). Applied Biochemistry and Biotechnology, 168, 928–935.
Yen, G. G., & Chen, H. Y. (1995). Journal of Agricultural and Food Chemistry, 43, 27–32.
DeGraft-Johnson, J., Kolodziejczyk, K., Krol, M., Nowak, P., Krol, B., & Nowak, D. (2007). Basic & Clinical Pharmacology & Toxicology, 100, 345–352.
Kuda, T., Tsunekawa, M., Hishi, T., & Araki, Y. (2005). Food Chemistry, 89, 617–622.
Moreles, F. J., & Jiménez-Pérez, S. (2001). Food Chemistry, 72, 119–125.
Rizzi, G. P. (2004). Journal of Agricultural and Food Chemistry, 52, 953–995.
Sumaya-Martinez, M. T., Thomas, S., Linard, B., Binet, A., & Guerard, F. (2005). Food Research International, 38, 1045–1050.
Bell, L. N. (1997). Food Chemistry, 59, 143–147.
Hu, H., Chen, W., Shi, Y., & Cong, W. (2006). Marine Pollution Bulletin, 52, 756–760.
Acknowledgments
This work was supported by the Salt Science Research Foundation (Grant No. 0740), Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuda, T., Yano, T. Mineral Composition of Seawater Bittern Nigari Products and Their Effects on Changing of Browning and Antioxidant Activity in the Glucose/Lysine Maillard Reaction. Appl Biochem Biotechnol 172, 2989–2997 (2014). https://doi.org/10.1007/s12010-013-0722-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0722-0