Abstract
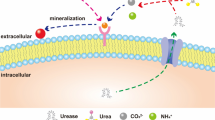
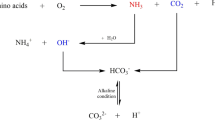
Contamination by Cd is a significant environmental problem. Therefore, we examined Cd removal from an environmental perspective. Ureolysis-driven calcium carbonate precipitation has been proposed for use in geotechnical engineering for soil remediation applications. In this study, 55 calcite-forming bacterial strains were newly isolated from various environments. Biomineralization of Cd by calcite-forming bacteria was investigated in laboratory-scale experiments. A simple method was developed to determine the effectiveness of microbially induced calcite precipitation (MICP). Using this method, we determined the effectiveness of biomineralization for retarding the flow of crystal violet through a 25-mL column. When the selected bacteria were analyzed using an inductively coupled plasma optical emission spectrometer, high removal rates (99.95 %) of Cd were observed following incubation for 48 h. Samples of solids that formed in the reaction vessels were examined using a scanning electron microscope. The CdCO3 compounds primarily showed a spherical shape. The results of this study demonstrate that MICP-based sequestration of soluble heavy metals via coprecipitation with calcite may be useful for toxic heavy metal bioremediation.







Similar content being viewed by others
References
Li, M., Cheng, X., & Guo, H. (2013). International Biodeterioration and Biodegradation, 76, 81–85.
Yavuz, Ö., Guzel, R., Aydin, F., Tegin, I., & Ziyadanogullari, R. (2007). Polish Journal of Environmental Studies, 16, 467–471.
Boquet, E., Boronat, A., & Cormenzana, A. R. (1973). Nature, 246, 527–529.
Rivadeneyra, M. A., Delgado, R., Moral, A. D., Ferrer, M. R., & Cormenzana, A. R. (1993). FEMS Microbiology Ecology, 13, 197–204.
Whiffin, V. S., Paassen, L. A., & Harkes, M. P. (2007). Geomicrobiology Journal, 24, 417–423.
Hammes, F., & Verstraete, W. (2002). Reviews in Environmental Science and Biotechnology, 1, 3–7.
Stocks-Fischer, S., Galinat, J. K., & Bang, S. S. (1999). Soil Biology & Biochemistry, 31, 1563–1571.
Chun, J., Lee, J. H., Jung, Y., Kim, M., Kim, S., & Kim, B. K. (2007). International Journal of Systematic and Evolutionary Microbiology, 57, 2259–2261.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). Molecular and Biological Evolution, 28, 2731–2739.
Natarajan, K. R. (1995). Journal of Chemical Education, 73, 556–557.
Hammes, F., Boon, N., De Villiers, J., Verstraete, W., & Siciliano, S. D. (2003). Applied and Environmental Microbiology, 69, 4901–4909.
Stahler, M. F., Ganter, L., Katherin, L., Manfred, K., & Stephen, B. (2005). FEMS Immunology and Medical Microbiology, 44, 183–189.
Park, I. S., & Hausinger, R. P. (1995). Science, 267, 1156–1158.
Qian, C., Wang, R., Cheng, L., & Wang, J. (2010). Chinese Journal of Chemistry, 28, 847–857.
DeJong, J. T., Mortensen, B. M., Martinez, B. C., & Nelson, D. C. (2010). Ecological Engineering, 36, 197–210.
Hamidi, A. A., Mohd, N. N., & Kamar, S. A. (2008). Bioresource Technology, 99, 1578–1583.
Sanchez, A. G., & Ayuso, E. A. (2002). Minerals Engineering, 15, 539–547.
Luo, S., Xiao, X., Xi, Q., Wan, Y., Chen, L., Zeng, G., et al. (2011). Journal of Hazardous Materials, 190, 1079–1082.
Cheung, K. H., Lai, H. Y., & Gu, J. D. (2006). Journal of Microbiology and Biotechnology, 16, 855–862.
Cheung, K. H., & Gu, J. D. (2007). International Biodeterioration & Biodegradation, 59, 8–15.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2011-0025229).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, CH., Han, SH., Shin, Y. et al. Bioremediation of Cd by Microbially Induced Calcite Precipitation. Appl Biochem Biotechnol 172, 1929–1937 (2014). https://doi.org/10.1007/s12010-013-0626-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0626-z