Abstract
The paper deals with the exploitation of Ipomoea carnea as a feedstock for the production of bioethanol. Dilute acid pretreatment under optimum conditions (3 %H2SO4, 120 °C for 45 min) produced 17.68 g L−1 sugars along with 1.02 g L−1 phenolics and 1.13 g L−1 furans. A combination of overliming and activated charcoal adsorption facilitated the removal of 91.9 % furans and 94.7 % phenolics from acid hydrolysate. The pretreated biomass was further treated with a mixture of sodium sulphite and sodium chlorite and, a maximum lignin removal of 81.6 % was achieved. The enzymatic saccharification of delignified biomass resulted in 79.4 % saccharification with a corresponding sugar yield of 753.21 mg g−1. Equal volume of enzymatic hydrolysate and acid hydrolysate were mixed and used for fermentation with a hybrid yeast strain RPRT90. Fermentation of mixed detoxified hydrolysate at 30 °C for 28 h produced ethanol with a yield of 0.461 g g−1. A comparable ethanol yield (0.414 g g−1) was achieved using a mixture of enzymatic hydrolysate and undetoxified acid hydrolysate. Thus, I. carnea biomass has been demonstrated to be a potential feedstock for bioethanol production, and the use of hybrid yeast may pave the way to produce bioethanol from this biomass.
Similar content being viewed by others
Introduction
In recent years, much research interest has been generated to produce bioethanol from a variety of renewable resources. The reasons behind the utilisation of these renewable resources for the production of alternative fuel are their abundant availability over earth's surface, low cost and potential to produce clean fuel [1]. The exploitation of these resources may provide a sustainable energy supply at local, regional and national levels. Lignocellulosic biomass is one of such renewable resources which can be used as potential feedstock for large-scale production of bioethanol and other forms of energy. The lignocellulosic biomass is mainly composed of cellulose, hemicellulose and lignin polymers. The cellulose and hemicellulose component of the biomass are the source of sugar substrates that can be fermented to ethanol [2]. The hydrolysate that is the fermentable sugar component can be derived from biomass either by acid or enzymatic hydrolysis, the later being the most favourable because the method is environmentally benign, efficient and involves lower energy requirement [3]. However, the major challenges in enzymatic hydrolysis are the reduction of cellulose crystallinity and the removal of lignin from biomass as they hinder the release of cellulose and hemicellulose to be hydrolysed [4, 5]. In this context, acid pretreatment is an effective and the most commonly used method for pretreatment which facilitates the yield of monomers of hemicellulosic sugars [6, 7]. Given the ability of hemicellulose to hydrolyse in acids, the acid pretreatment have been used widely for fractionating the components of lignocellulosic biomass [8]. Although the process is associated with the formation of toxic by-products that cause inhibitory effects on the microorganisms during fermentation [9], an effective detoxification method and/or the use of inhibitor tolerant yeast strain can make fermentation feasible [10, 11]. Detoxification using over-liming or activated charcoal adsorption has been the choice of many researchers for detoxification of these inhibitors [10, 12].
Furthermore, the maximum utilisation of pentose and hexose sugars present in lignocellulosic substrates is an important factor to make ethanol production commercially feasible. The conventional Saccharomyces cerevisiae yeast used for hexose fermentation is not able to ferment pentose sugars to ethanol. Therefore, the simultaneous conversion of pentose and hexose sugars to ethanol has been explored by the use of hybrid yeast strains [13]. In the present study, a multiple stress-tolerant hybrid yeast strain developed earlier with an ability to ferment glucose and xylose was used to ferment multiple sugar substrates present in biomass [14, 15].
Ipomoea carnea, commonly known as bush morning glory is a weed found abundantly in India, Brazil, the USA and other countries [16]. This amphibious plant is considered as one of the most productive of macrophytes as it grows profusely on water bodies and adjoining marshy lands [17]. The weed causes neurological disorders in livestock on consumption [18], thus it cannot be used as a cattle feed. The wild growth and abundance of this plant make it a cheaper and potential substrate for production of bioethanol.
In the present study, an effort has been made to exploit the I. carnea as lignocellulosic feedstock for the production of bioethanol. Biomasses widely differ in composition and even the same type of biomass may have different composition because of climatic conditions and seasonal variations. These variations in composition can have a significant impact on their conversion processes to bioethanol. Furthermore, to the best of our knowledge I. carnea is an unexploited plant species for the bioethanol production. Therefore, it is essential to explore suitable methods for its conversion to produce ethanol. In this study, an attempt has been made to investigate the influence of dilute acid pretreatment, enzymatic hydrolysis and fermentation to maximise the ethanol yield.
Materials and Methods
Microorganism and Enzymes
A mutated hybrid strain S. cerevisiae RPRT90 (GenBank accession number JN887371) with ability to ferment glucose and xylose simultaneously was used for this study. The hybrid strain was developed by fusion of protoplast of S. cerevisiae and Pachysolen tannophilus [14]. Furthermore, the strain was improved by multiple mutations for the tolerance to various stress factors like high ethanol concentrations, high temperature and fermentation inhibitors [15]. The strain RPRT90 was maintained on YPD agar slants and the inoculum for fermentation was prepared using 20.0 g L−1 glucose, 10.0 g L−1 xylose, 10.0 g L−1 yeast extract and 20.0 g L−1 peptone at pH 5.0 ± 0.2 and temperature 30 °C. Hydrolytic enzymes, cellulase from Trichoderma reesei (6.0 FPU/mg) and cellobiase from Aspergillus niger (250 U/mL) were purchased from Sigma-Aldrich Co. (St. Louis, USA).
Composition Analysis of Biomass
I. carnea biomass was collected from the campus of National Institute of Technology, Rourkela, India. The plants stems were cut near to root and the whole plant biomass including leaves, flowers and fruits were used for the experiment. The biomass was washed, dried in shade for 48 h and pulverised in the laboratory grinder (Bajaj Electricals, India) to the size range of 0.5 to 0.75 mm. The communited biomass was air-dried overnight at room temperature to remove left over moisture. The biomass was extracted in soxhlet apparatus with alcohol-benzene (solid loading 10 %, w/v) for 6 h. The structural carbohydrates, lignin and sugar monomer content of extractive free biomass were determined following the standard laboratory analytical procedures (LAPs) developed by National Renewable Energy Laboratory (NREL, USA) [19]. The proximate and ultimate analyses of biomass were done according to the methods described by Naik et al. [20]. All the experiments were performed in triplicates and the values are presented as mean.
Selection of Suitable Pretreatment Reagent
The I. carnea biomass was subjected to acid (sulphuric acid, hydrochloric acid and phosphoric acid), alkali (sodium hydroxide) and alkaline peroxide treatments. The purpose of the study was to find a suitable pretreatment reagent to increase the hemicellulose and lignin release from biomass. For acid and alkali pretreatments, the biomass was soaked in 1–4 % (v/v) pretreatment reagent at 10 % (w/v) solid content and autoclaved at 120 °C for 1 h. The slurry was filtered through double-layered muslin cloth and the biomass collected as residue was washed thoroughly with tap water till the pH reached 6.5–7.0. The neutralised biomass was dried at room temperature for 2 h and then stored at 4 °C in sealed packs until use. The hydrolysate was analysed for the amount of sugars and by-products like furan and phenolics.
The alkaline peroxide treatment was done according to the protocol described by Sun et.al [21]. In brief, biomass was treated with 2 % (v/v) H2O2 at 50 °C and pH 11.5 for 16 h. The insoluble residue was collected by filtration and the filtrate was analysed for sugar and phenolics released.
Biomass Pretreatment
The effects of major process parameters for biomass pretreatment such as temperature (100–140 °C) and time of treatment (30–60 min) were investigated to establish the optimum conditions for maximum release of sugars and minimum release of by-products. The variation in the sugar content of acid hydrolysate was studied with respect to the varying pretreatment severity. The pretreatment severity was determined by the combined severity factor (CSF) using the formula reported by Lloyd and Wyman [22]. The CSF was calculated as
where t is reaction time in minutes, T H is the reaction temperature (in degrees Celsius), T R is the reference temperature (100 °C), and pH is the acidity of the dilute acid.
Detoxification of Acid Hydrolysate
The acid hydrolysate was detoxified using overliming and activated charcoal treatment both individually and in combination. Overliming was performed by the addition of calcium hydroxide to the acid hydrolysate till pH reached 10.0. The whole mixture was stirred for 30 min at 45 °C and allowed to cool to room temperature. The acid hydrolysate was then neutralised with concentrated H2SO4 and centrifuged at 10,000×g for 10 min. The supernatant was mixed with 2.0 % (w/v) activated charcoal and the slurry was stirred continuously for 90 min at room temperature. Finally, the detoxified sugar syrup was recovered by vacuum filtration. All the experiments were performed in triplicates and the values are presented as mean.
Delignification of Pretreated Biomass
Delignification of the pretreated biomass was carried out according to the method described by Kuhad et al. [10]. The acid pretreated residue was treated with 5–20 % (w/v) sodium sulphite alone and in combination with sodium chlorite. The solid loading of biomass was 10 % (w/v). The mixture was autoclaved at different temperatures (100–140 °C) and time intervals (30 and 45 min). The mixture was then filtered and the residue was washed till neutral pH is obtained and stored at 4 °C until further use. The lignin content in the delignified biomass was estimated and the percentage lignin removal was calculated.
Enzymatic Hydrolysis
The enzymatic hydrolysis of the delignified cellulosic residue was carried out in 500 mL Erlenmeyer flasks. The delignified biomass was added to 0.05 M citrate phosphate buffer (pH 5.0) with 6.5 % solid content (w/v). Cellulase (1.5–3.75 FPU/mL) and cellobiase (three times of the cellulase concentration) were added to the suspension and incubated in a controlled environment incubator shaker (CIS-24 BL, Remi Instruments Ltd., India) at 50 °C and 150 rpm for varying time intervals (8–40 h). The effect of surfactants on enzymatic saccharification was studied by adding different chemical surfactants like Tween 20 (T20), polyethylene glycol (PEG) and Tween 80 (T80) at varying concentrations (0.25–1.25 %, w/v or v/v). A study on supplementation of bovine serum albumin (BSA) with chemical surfactants was conducted by adding 0.25 % (w/v) BSA (Sigma-Aldrich, St. Louis, MO, USA) 1 h prior to the addition of enzymes and surfactants. Samples were withdrawn after every 4-h interval and analysed for reducing sugar released in the reaction mixture. All the experiments were performed in triplicates, and error bars shown in the results are standard deviations of triplicates.
Fermentation of Mixed Hydrolysate
Equal volume (2 L) of detoxified acid hydrolysate and enzymatic hydrolysate were mixed along with the supplementation of 3.0 g L−1 yeast extract, 2.0 g L−1 KH2PO4, 1.0 g L−1 peptone, 0.5 g L−1 MgSO4·7H2O, 0.5 g L−1 NH4Cl, 0.25 g L−1 (NH4)2HPO4, 0.1 g L−1 CaCl2·2H2O and 0.1 g L−1 FeCl3.2H2O. The fermentation of mixed hydrolysate (4 L) was conducted in a 5-L laboratory bench-top fermenter (Biostat B Plus, Sartorius India Ltd., India) using 10 % inoculum of hybrid yeast strain RPRT90. The fermentation conditions like temperature, 30 °C; pH 5.5; agitation, 150 rpm; and aeration, 0.35 L/min were maintained throughout the experiment. The fermentation broth was centrifuged and used for ethanol, residual sugar and biomass estimation.
Analytical Methods
Ethanol was enzymatically estimated as per the method described by Puria et al. [23]. In this assay, fermentation broth was centrifuged and the supernatant was diluted ten times with 50 mM sodium pyrophosphate buffer (pH 8.8); 0.65 mL sodium pyrophosphate buffer (50 mM), 0.75 mL β-NAD (15 mM) and 50 μL alcohol dehydrogenase enzyme (3.0 U) were added to 50 μL diluted sample and optical density at 340 nm was measured using spectrophotometer. BSA was used in place of enzyme in control. The ethanol concentration in the sample was calculated using a standard graph. The estimation of sugars in hydrolysate and biomass were determined following the standard LAPs developed by NREL using HPLC (Shimadzu Corp., Kyoto, Japan) with an Aminex HPX-87H organic analysis column (300 × 7.8 mm; Bio-Rad Hercules, CA) and a refractive index detector [19]; 4 mM H2SO4 was used as eluent and flow rate was maintained at 0.65 mL/min at 65 °C. Total phenolics released were determined by Folin–Ciocalteu reagent method using vanillin as standard [24]. The optical density (A 600 nm) of fermentation broth was measured using a double-beam UV–visible spectrophotometer (Systronics India Ltd., Bangalore, India). Total furan content in hydrolysate was estimated by a spectrophotometric method based on the difference in absorbance at 284 and 320 nm before and after the pretreatment [25]. The biomass yield of yeast cells were measured by dry cell mass weight measurement. The percentage theoretical yield of ethanol was calculated as
Results and Discussion
Biomass Composition Analysis
The biomass of I. carnea was found to contain cellulose, hemicellulose and lignin as major components. The biomass contained 2.13 ± 0.51 % benzene-ethanol extractives, 49.6 ± 2.05 % cellulose, 16.4 ± 0.97 % hemicelluloses and 24.8 ± 0.74 % lignin. The high carbohydrate content (66.5 %) of I. carnea biomass makes it a potential source for bioethanol production. The sugar monomer content was determined as 56.21 ± 0.37 % glucose, 20.3 ± 0.74 % xylose, 14.58 ± 0.84 % arabinose and 10.9 ± 0.57 % other sugars. The results of proximate and ultimate analysis of the biomass are represented in Table 1.
Selection of Suitable Pretreatment Reagent
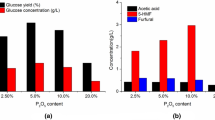
A comparative study was done on different pretreatment reagents like acids, alkali and alkaline peroxide to verify their efficiency in releasing sugars. Among the three different acids used, sulphuric acid was found to release higher amount of sugars with respect to phosphoric and hydrochloric acid. The maximum yield of hemicellulosic sugars was obtained using 3 % sulphuric acid (174.88 mg g−1) followed by 4 % sulphuric acid (153.42 mg g−1) and 3 % hydrochloric acid (138.76 mg g−1) (Fig.1a). Sulphuric acid was also found to be more efficient in pretreating I. carnea biomass than NaOH and alkaline peroxide. The hemicellulosic sugar yield obtained using sulphuric acid was 41.5 and 13.2 % higher than the yield using NaOH and alkaline peroxide. The use of NaOH as pretreatment reagent resulted in enhanced release of phenolics but a simultaneous reduction in the sugars release was also observed. The loss of fermentable sugars and production of inhibitory compounds with the use of NaOH was also reported previously [26]. The pretreatment of biomass using oxidising compound like alkaline peroxide was accompanied with reduction in both sugar and phenolics release. According to a report published by Hendricks et al., during an oxidative pretreatment often a lot of cellulose and hemicellulose get lost, because of non-selective oxidation [26]. Our results on acid and alkaline pretreatment are well in accordance with the previous reports of Pasha et al. on Lantana camara biomass. As among all the reagents studied the use of sulphuric acid was found to be most favourable for pretreatment of I. carnea biomass, sulphuric acid was selected for further pretreatment parameters studies.
Furthermore, as the severity of pretreatment conditions is reported to effect the enzymatic saccharification of the pretreated biomass [27], the saccharification yield of the pretreated biomass achieved using various acid treatments were also compared as shown in Fig. 1b. The saccharification yield of the pretreated biomass was determined after sulphite delignification and enzymatic hydrolysis. The hydrolysis of the pretreated biomass for determination of the saccharification yield was done using 2.25 FPU/mL cellulase, 7 U/mL β-glucosidase and T20 as surfactant (1 %, v/v). The saccharification yield of the pretreated biomass was severely affected by the use of hydrochloric acid as compared with other two acids used under study. The maximum saccharification yield was found using 3 % sulphuric acid (648.69 mg g−1 dry substrate) and comparable yield was obtained using 4 % sulphuric acid (640.61 mg g−1) and 3 % phosphoric acid (640.35 mg g−1). The saccharification yield obtained by using alkaline peroxide and sodium hydroxide as pretreatment reagent were comparatively low as both of these reagents are reported to cause non-selective degradation of sugars. Based on the sugar yield of acid hydrolysate and saccharification yield of biomass pretreated with different acids, 3 % dilute sulphuric acid has been established as the most effective reagent for the pretreatment of I. carnea biomass and thus 3 % sulphuric acid was used as pretreatment reagent for further study.
Optimisation of Dilute Sulphuric Acid Pretreatment
Besides the selection of most optimum reagent and its concentration, as described in previous section, the effects of other important pretreatment parameters such as retention time (30–60 min) and temperature (100–140 °C) on the constituents of acid hydrolysate were investigated using 3 % sulphuric acid to establish the most favourable pretreatment condition. The range of pretreatment time and temperature were chosen on the basis of the results of previous reports on dilute sulphuric acid pretreatment of various lignocellulosic substrates [28–30]. The acid hydrolysis of the biomass results in the formation of a variety of degradation products like pentose sugars degrade to furfural, hexoses to HMF and lignin degrades to phenolics. These degradation products affect the yeast growth and ethanol fermentation by reducing the activities of various enzymes such as alcohol dehydrogenase and pyruvate dehydrogenase [31]. Thus, a simultaneous study on the effect of pretreatment severity conditions on the release of fermentation inhibitors was performed. From the experimental results presented in Table 2, the xylose content in acid hydrolysate increased with increase in temperature from 80 to 120 °C and then decreased afterwards. Similarly, increase in incubation time from 30 to 45 min favoured the sugar release but further incubation resulted in reduction in sugar yield. The maximum xylose yield of 17.68 g L−1 was obtained when the biomass was pretreated at 120 °C and 45 min treatment. The corresponding severity index is calculated as 1.37. Further increase in severity showed detrimental effect on xylose yield. The release of glucose in the acid hydrolysate also showed a similar trend with maximum glucose (3.71 g L−1) release at severity index of 1.5 while the total sugar in the acid hydrolysate, including both xylose and glucose was maximum at CSF 1.5 (21.03 g L−1) followed by CSF 1.37 (20.95 g L−1). The phenolics and furan yield increased with the increase in severity and the maximum phenolics (1.052 g L−1), and furans (1.92 g L−1) were generated by treatment at 140 °C for 60 min. The increase in phenolics release and decrease in sugar yield at high temperature and incubation time was also reported by Kuhad et al. using L. camara biomass [10]. The saccharification yield of the pretreated substrate was observed to be in its maximum at 120 °C and 60 min (651.78 mg g−1) and a comparable yield was also obtained at 120 °C and 45 min (648.21 mg g−1). The saccharification yield also decreased with the further increase in severity. The decrease in sugar contents at high severity conditions may be due to the degradation of sugars to furans (furfural and hydroxyl-methyl furfurals) under severe conditions. Considering both sugar yield of acid hydrolysate and saccharification yield of biomass, the acid pretreatment at 120 °C and 45 min using 3 % sulphuric acid was found to be most optimum for I. carnea biomass.
Detoxification of Acid Hydrolysate
A variety of toxic by-products are evidently formed during acid pretreatment, and these by-products inhibit the yeast growth and fermentation. Therefore, detoxification of acid hydrolysate is necessary to avoid the adverse effect caused by various inhibitors. Different methods have been investigated for the removal of fermentation inhibitory compounds, however, the overliming and activated charcoal adsorption are the most commonly used methods [10, 12]. In the present study, the detoxification of acid hydrolysate was performed using overliming and activated charcoal treatment, both individually and in combination. The raising of pH using calcium hydroxide results in transformation of the inhibitory compounds and the use of activated charcoal effectively removes the hydrophobic inhibitory compounds. Among these two methods, overliming was found to be more efficient in removing furan and phenolics as compared with activated charcoal treatment. However, the maximum removal of inhibitors was observed using the combination of both methods. From Table 3, the detoxification of acid hydrolysate by overliming was found to reduce the content of inhibitors while further treatment with activated charcoal resulted in almost complete removal of inhibitors. The concentration of phenolics was reduced to 0.054 ± 0.012 from 1.02 ± 0.041 g L−1, while furan from 1.13 g L−1 to 0.091 g L−1. The corresponding percentage removal of phenolics and furans were calculated as 94.7 and 91.9 %, respectively. In the present study, the combination of overliming and activated charcoal treatment was found to be very effective in removing the inhibitory compound from I. carnea acid hydrolysate. Furthermore, a small but significant reduction in sugar content (9.1 %) was also observed to be associated with the detoxification process. The loss of sugar of 9.3 and 7.2 % was also reported earlier with sunflower hull and L. camara biomass respectively [10, 32]. Furthermore, the efficiency of the fermentability of the detoxified and undetoxified hydrolysate was studied and a marginal increase (14.3 %) in ethanol concentration was obtained with detoxification (Table 3). In earlier studies with sugarcane bagasse and corn cob, an improvement in ethanol yield of 58 and 90 % on overliming and activated charcoal detoxification were earlier reported by Martin et al. and Ge et al., respectively [33, 34]. However, in the present study, the improvement in fermentability after detoxification is less compared with earlier reports. This result may be attributed to the fact that the yeast strain RPRT90 used for fermentation in this study is tolerant to high concentrations of various toxic inhibitors like furfural and acetic acid.
Delignification of Pretreated Biomass
During acid treatment, though a certain percentage of lignin is removed, most of the lignin remains intact to the cellulosic substrate. The presence of lignin prevents the accessibility of organic components of biomass to the hydrolysing enzyme and hence an appropriate delignification treatment of biomass is essential. Various delignification approaches have been exploited in past few decades like alkaline peroxide pretreatment [35], sodium chlorite pretreatment [28] and sulphite pretreatment [10]. In the present study, delignification of the acid pretreated biomass was carried out at varying concentrations of sodium sulphite both alone and in combination with sodium chlorite. As represented in Fig. 2, a steady increase in the percentage lignin removal was observed with increase in the concentration of sodium sulphite from 10 to 20 % (w/v) and sodium sulphite concentration beyond 20 % did not show any benefit in percentage lignin removal. Furthermore, the percentage lignin removal was also observed to increase with increase in temperature and incubation time up to 140 °C and 45 min. The maximum percentage of lignin removal of 75.6 % (251.7 mg/g) was obtained using 20 % sodium sulphite at 140 °C and 45 min. Further study on delignification with supplementation of sodium sulphite with sodium chlorite showed a major enhancement in phenolics yield and hence the lignin removal. Moreover, supplementation with sodium chlorite also decreased the requirement of sodium sulphite from 20 to 10 %. Figure 2 shows that the treating of I. carnea biomass with 10 % sodium sulphite and 3 % sodium chlorite was found to be most favourable for lignin removal and the percentage lignin removal of 81.6 % was achieved. Similar study has been reported with L. camara by Kuhad et al. As reported earlier, sodium chlorite on heating produces chlorine dioxide that helps in depolymerisation of lignin and swelling of biomass.
Enzymatic Hydrolysis of Delignified Biomass
The cellulase and β-glucosidase (cellobiase) enzymes are reported to hydrolyse the cellulose polymers to free glucose monomeric units. The cellulase catalyses the cleavage of internal bonds of cellulose chain to short chains and β-glucosidase act on the cello-oligosaccharides and cellobiose to release glucose monomers [36]. The hydrolysis of delignified biomass was performed to convert the cellulosic polymer to the monomeric glucose units using cellulase and β-glucosidase enzyme. The various parameters of enzymatic saccharification process like enzyme loading and reaction time were optimised to improve the cellulose-enzyme reactions. Furthermore, the use of surfactant has a great effect on the saccharification process as it inhibits non-productive attachment of exoglucanase to lignin surface and thus allows greater access of saccharifying exoglucanae to cellulose, resulting in high yield of sugar [5]. Therefore, the influences of various surfactants and their varying concentrations on the release of sugar during hydrolysis were also investigated. As represented in Fig. 3, the saccharification yield was found to increase steadily with increase in surfactant concentration up to 0.75 % and further increase in surfactant concentration showed a detrimental effect on sugar yield. The phenomenon of the reduction in enzyme activity at high surfactant concentration may be attributed to the formation of reverse micelles as reported by Chen et al. [37]. Among the various surfactants used, the highest saccharification yield (708.6 ± 4.2 mg g−1) was obtained with the addition of T20 as surfactant and a comparable yield (689.2 ± 2.6 mg g−1) was also released using T80. In this study, PEG was found to be less efficient surfactant as compared with T20 and T80. Furthermore, a study was performed on the effect of the addition of a non-hydrolysing protein, BSA on enzymatic hydrolysis. BSA was added 1 h before addition of surfactants and enzymes. Figure 3 shows that a further improvement in saccharification yield up to 738.2 mg g−1 was achieved by the supplementation of BSA with T80. The non-hydrolytic proteins like BSA bind to the cellulose and result in swelling of microfibrils, thereby decreasing crystallinity and increasing enzyme accessibility [36]. Overall, an increase of 31.0 % in sugar yield was observed with the combination of T80 and BSA. Though no significant increase in sugar yield was reported by the addition of BSA to T20 by Erikkson et al. [38], in the present research, the addition of BSA before 1 h of addition of enzymes and surfactant was found to be effective in enhancing enzymatic saccharification of lignocellulosic biomass.
Enzyme concentration is considered to be one of the major factors affecting the conversion of cellulose to glucose monomers. As shown in indicated Fig. 4, the increase in cellulase concentration from 1.5 to 3.0 FPU/mL was found to be effective in enhancing sugar release. The maximum saccharification yield was obtained with 3.0 FPU/mL of cellulase (753.21 mg g−1), and the enzyme concentration above 3.0 FPU was observed to be unfavourable for enzymatic hydrolysis. This phenomenon can be explained by the occurrence of feedback inhibition of glucose over the action of cellulase. The results of varying enzyme concentrations were in good agreement with the previous studies of enzymatic saccharification of sugarcane tops [39] and barley straw [40]. Furthermore, the time course study of the enzymatic hydrolysis showed that the saccharification yield increased steadily with time up to 32 h of hydrolysis and the yield remained same even on prolonged incubation. However, with higher cellulase concentration (3.75 FPU/mL), the saccharification yield increased up to 24 h of incubation and then it remained constant thereafter. The maximum saccharification efficiency (79.4 %) achieved in the present study is in agreement with the earlier reports on saccharification efficiency of 77.7 and 78.7 % from L. camara [10, 30] and 82.8 % from Prosopis juliaflora biomass [29].
Ethanol Fermentation
Fermentation of the Mixture of Enzymatic Hydrolysate and Detoxified Acid Hydrolysate
The ethanol fermentation of the mixed hydrolysate was carried out in a 5-L bench-top fermenter. The acid and enzymatic hydrolysates of I. carnea were mixed in equal amount (2 L) and fermentation was carried out using the hybrid strain RPRT90. The fermentation profile is shown in Fig. 5. It is observed from the experimental results that the fermentation of mixed hydrolysate containing the detoxified acid hydrolysate (18.69 ± 0.54 g L−1 sugar) and enzymatically hydrolysed cellulosic hydrolysate (53.42 ± 0.94 g L−1 sugars) produced 29.01 ± 0.32 g L−1 ethanol after 28-h incubation. Out of 72.11 ± 0.62 g L−1 of sugar, 62.82 ± 0.32 g L−1 was consumed during ethanol production and 9.29 ± 0.45 g L−1 was left unutilised which contained 3.84 ± 0.27 g L−1 of glucose and 5.45 ± 0.83 g L−1 of pentose sugars. The maximum ethanol productivity of 1.03 g L−1 h−1, ethanol yield of 0.461 g g−1, percent sugar conversion of 87.1 %, and percent theoretical yield of 90.3 % were obtained after 28 h of fermentation. A decline in ethanol production was observed beyond 28 h which may be due to the consumption of accumulated ethanol by the yeast [41]. The yeast biomass yield increased with increase in time and maximum biomass yield of 0.463 g g−1 was obtained within 28 h of fermentation. The biomass yield was observed to remain constant thereafter.
Fermentation of the Mixture of Enzymatic Hydrolysate and Undetoxified Acid Hydrolysate
The fermentation profile of the undetoxified hydrolysate and enzymatic hydrolysate is shown in Fig. 6. The sugar content of the mixed hydrolysate was found to be 74.45 ± 1.67 g L−1 containing 21.03 ± 0.73 g L−1 sugar obtained from undetoxified acid hydrolysate and 53.42 ± 0.94 g L−1 sugars from enzymatically hydrolysed cellulosic hydrolysate. The fermentation of total sugar produced 24.23 ± 0.36 g L−1 ethanol after 28 h incubation by utilising 58.46 ± 0.63 gL-.1 of sugar. The percent sugar conversion was calculated as 78.5 % while 15.99 ± 0.56 g L−1 was left unutilised comprising of 4.29 ± 0.43 g L−1 of glucose and 11.7 ± 0.37 g L−1 of pentose sugars. The maximum ethanol yield and ethanol productivity achieved after 28 h of fermentation were 0.414 g g−1 and 0.865 g L−1 h-1 and the percent theoretical yield was calculated as 81.1 %. Furthermore, increase in fermentation time resulted in a decline in ethanol production. The biomass yield increased with increase in time up to 28 h, reached a maximum biomass yield of 0.45 g g−1 and no further increase in biomass was observed with increase in fermentation time (Fig. 6).
The ethanol produced by RPRT90 from mixed hydrolysate containing detoxified acid hydrolysate (27.2 g L−1) was slightly higher than the undetoxified hydrolysate (23.05 g L−1). In the present study, the improvement in fermentability with detoxification is not much significant. This may be because the strain RPRT90 is tolerant to fermentation inhibitors like furfural and acetic acid. Therefore, it is also established that the strain RPRT90 can be used to ferment acid I. carnea hydrolysate even without detoxification.
Similar work on fermentation of mixed hydrolysates using hybrid yeasts has been reported earlier by few researchers. The fermentation of mixed hydrolysate of L. camara biomass using mutant hybrid yeast CP11 was also reported by Pasha et al. [30]. The hybrid CP11 gave an ethanol yield of 0.431 ± 0.012 g g−1 and productivity of 0.67 ± 0.015 g L−1 h−1. The yield (0.46 g g−1) obtained in the present study using hybrid yeast RPRT90 is higher than CP11. Furthermore, a study on the fermentation of mixed hydrolysate of Prosopis juliflora biomass using mutant hybrid yeast CP11developed from S. cerevisiae and Candida shehatae was also reported by Pasha et al. [30]. In their study, the fusant mutant yeast CP11 gave an ethanol yield of 0.459 g/g, productivity of 0.67 g L−1 h-1 and fermentation efficiency of 90 %. The ethanol yield obtained in the present study is in good agreement with the yield reported by Pasha et al. In another study, the separate fermentation of acid and enzymatic hydrolysate of L. camara biomass using Pichia stipitis and S. cerevisiae was performed by Kuhad et al. [10]. They reported an ethanol production of 22.46 g L−1 which is also considerably lower than the ethanol obtained in the present study with RPRT90 (25.45 g L−1). The ethanol yields obtained from acid and enzymatic hydrolysate reported by Kuhad et al. were 0.32 and 0.48 g g−1. Thus it has been established that RPRT90 is a potential yeast strain for bioethanol production and even has shown better activity than the activity shown by other mutant hybrid strains reported.
Conclusions
The exploitation of cheaper, abundant and renewable resources is vital for the production of bioethanol as an alternative fuel for transportation sector. In this study, it has been demonstrated that I. carnea biomass consisting of high percentage of carbohydrates can be a potential feedstock for bioethanol production through the establishment of effective conversion methods like pretreatment (acid pretreatment, detoxification and delignification), hydrolysis (enzymatic saccharification) and fermentation. Furthermore, the use of the multiple stress tolerant hybrid yeast strain that has the ability to convert hexose and pentose sugars simultaneously may pave the way to produce bioethanol from lignocellulosic biomass commercially.
References
Balat, M., Balat, H., & Oz, C. (2008). Progress in Energy and Combustion Science, 34, 551–573.
Sreenath, H. K., & Jeffries, T. W. (2000). Bioresource Technology, 72, 253–260.
Balat, M. (2011). Energy Conversation and Management, 52, 858–875.
Yoshida, M., Liu, Y., Uchida, S., Kawarada, K., Ukagami, Y., Ichinose, H., et al. (2008). Bioscience Biotechnology and Biochemistry, 72, 805–810.
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y. Y., Holtzapple, M., et al. (2005). Bioresource Technology, 96, 673–686.
Kim, S. B., Lee, J. H., Oh, K. K., Lee, S. J., Lee, J. Y., Kim, J. S., et al. (2011). Biotechnology and Bioprocess Engineering, 16, 725–732.
Singh, S., Simmons, B. A., & Vogel, K. P. (2009). Biotechnology and Bioengineering, 104, 68–75.
Lee, Y. Y., Iyer, P., & Torget, R. W. (1999). Advances in Biochemical Engineering/Biotechnology, 65, 93–115.
Hendriks, A. T. W. M., & Zeeman, G. (2009). Bioresource Technology, 100, 10–18.
Kuhad, R. C., Gupta, R., Khasa, Y. P., & Singh, A. (2010). Bioresource Technology, 101, 8348–8354.
Huang, C.-F., Lin, T.-H., Guo, G.-L., & Hwang, W.-S. (2009). Bioresource Technology, 100, 3914–3920.
Mussatto, S. I., Santos, J. C., & Roberto, I. C. (2004). Journal of Chemical Technology and Biotechnology, 79, 590–596.
Ho, N. W., Chen, Z., & Brainard, A. P. (1998). Applied and Environmental Microbiology, 64, 1852–1859.
Kumari, R., & Pramanik, K. (2012). Applied Biochemistry and Biotechnology, 167, 873–884.
Kumari, R., & Pramanik, K. (2012). Journal of Bioscience and Bioengineering, 114, 622–629.
Patel, A. K., Singh, V. K., Yadav, R. P., Moir, A. J., & Jagannadham, M. V. (2009). Phytochemistry, 70, 1210–1216.
Ganesh, P. S., Sanjeevi, R., Gajalakshmi, S., Ramasamy, E. V., & Abbasi, S. A. (2008). Bioresource Technology, 99, 812–818.
Schwarz, A., Gorniak, S. L., Bernardi, M. M., Dagli, M. L., & Spinosa, H. S. (2003). Neurotoxicology and Teratology, 25, 615–626.
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D, et al. (2008) http://www.nrel.gov/biomass/analytical_procedures.html
Naik, S., Goud, V. N., Rout, P. K., Jacobson, K., & Dalai, A. K. (2010). Renewable Energy, 35, 1624–1631.
Sun, X. F., Xu, F., Sun, R., Fowler, P., & Braid, M. S. (2005). Carbohydrate Research, 340, 97–106.
Lloyd, T. A., & Wyman, C. E. (2005). Bioresource Technology, 96, 1967–1977.
Puria, R., Mannan, M. A., Chopra-Dewasthaly, R., & Ganesan, K. (2009). FEMS Yeast Research, 9, 1161–1171.
Chandler, S. F., & Dodds, J. H. (1983). Plant Cell Reports, 2, 105–108.
Martinez, A., Rodriguez, M. E., York, S. W., Preston, J. F., & Ingram, L. O. (2000). Biotechnology Progress, 16, 637–641.
Hendricks, T. W. M., & Zeeman, G. (2009). Bioresource Technology, 100, 10–18.
Kabel, M. A., Bos, G., Zeevalking, J., Voragen, A. G. J., & Schols, H. A. (2007). Bioresource Technology, 98, 2034–2042.
Guo, G. L., Chen, W. H., Chen, W. H., Men, L. C., & Hwang, W. S. (2008). Bioresource Technology, 99, 6046–6053.
Gupta, R., Sharma, K. K., & Kuhad, R. C. (2009). Bioresource Technology, 100, 1214–20.
Pasha, C., Nagavalli, M., & Rao, L. V. (2007). Letters in Applied Microbiology, 44, 666–672.
Modig, T., Liden, G., & Taherzadeh, M. J. (2002). Biochemical Journal, 363, 769–776.
Okur-Telli, M., & Saracoglu-Eken, N. (2008). Bioresource Technology, 99, 2162–2169.
Martín, C., Marcet, M., Almazán, O., & Jonsson, L. J. (2007). Bioresource Technoogy, 98, 1767–1773.
Ge, J.-P., Cai, B.-Y., Liu, H.-Z., Fang, B.-Z., Song, G., Yang, X.-F., & Ping, W.-X. (2011). African Journal of Microbiology Research, 5(10), 1163–1168.
Sun, R. C., Tomkinson, J., Wang, S. Q., & Zhy, W. (2000). Polymer Degradation and Stability, 67, 101–109.
MacLellan, J. (2010). Basic Biotechnology, 6, 31–35.
Chen, N., Fan, J. B., Xiang, J., Chen, J., & Liang, Y. (2006). BBA-Proteins Proteomics, 1764, 1029–1035.
Eriksson, T., Borjesson, J., & Tjerneld, F. (2002). Enzyme and Microbial Technology, 31, 353–364.
Sindhu, R., Kuttiraja, M., Binod, P., Janu, K. U., Sukumaran, R. K., & Pandey, A. (2011). Bioresource Technology, 102, 10915–10921.
Kim, H. Y., Lee, J. W., Jeffries, T. W., & Choi, I. G. (2011). Bioresource Technology, 102, 1440–1446.
Ramon-portugal, F., Pingaud, H., & Strehaiano, P. (2004). Biotechnology Letters, 26, 1671–1674.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumari, R., Pramanik, K. Bioethanol Production from Ipomoea Carnea Biomass Using a Potential Hybrid Yeast Strain. Appl Biochem Biotechnol 171, 771–785 (2013). https://doi.org/10.1007/s12010-013-0398-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0398-5