Abstract
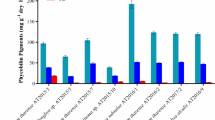
Zeaxanthin carotenoids are class of commercially important natural products and diverse biomolecules produced by plants and many microorganisms. Bacteria often produce a cocktail of polar and nonpolar carotenoids limiting their industrial applications. Marine members of the family Flavobacteriaceae are known to produce potential carotenoids such as astaxanthin and zeaxanthin. A few bacterial species have been reported for the predominant production zeaxanthin. Here, we report the molecular identification of the zeaxanthin as a major carotenoid produced by two novel bacteria (YUAB-SO-11 and YUAB-SO-45) isolated from sandy beaches of South West Coast of India and the effect of carbon sources on the production of zeaxanthin. The strains were identified based on the 16S rRNA gene sequencing as a member of genus Muricauda. The closest relatives of YUAB-SO-11 and YUAB-SO-45 were Muricauda aquimarina (JCM 11811T) (98.9 %) and Muricauda olearia (JCM 15563T) (99.2 %), respectively, indicating that both of these strains might represent a novel species. The highest level of zeaxanthin production was achieved (YUAB-SO-11, 1.20 ± 0.11 mg g−1) and (YUAB-SO-45, 1.02 ± 0.13 mg g−1) when cultivated in marine broth supplemented with 2 % NaCl (pH 7) and incubated at 30 °C. Addition of 0.1 M glutamic acid, an intermediate of citric acid cycle, enhanced the zeaxanthin production as 18 and 14 % by the strains YUAB-SO-11 and YUAB-SO-45 respectively. The zeaxanthin showed in vitro nitric oxide scavenging, inhibition of lipid peroxidation, and 2,2-diphenyl-1-picryl hydrazyl scavenging activities higher than the commercial zeaxanthin. The results of this study suggest that two novel strains YUAB-SO-11 and YUAB-SO-45 belonging to genus Muricauda produce zeaxanthin as a predominant carotenoid, and higher production of zeaxanthin was achieved on glutamic acid supplementation. The pigment showed good in vitro antioxidant activity, which can be exploited further for commercial applications.






Similar content being viewed by others
References
Augusti, P. R., Conterato, G. M. M., Somacal, S., Sobieski, R., Quatrin, A., Maurer, L., et al. (2009). Astaxanthin reduces oxidative stress, but not aortic damage in atherosclerotic rabbits. Journal of Cardiovascular Pharmacology and Therapeutics, 14, 314–322.
Bhosale, P., & Bernstein, P. S. (2005). Microbial xanthophylls. Applied Microbiology and Biotechnology, 68, 445–455.
Asker, D., Awad, T. S., Beppu, T., & Ueda, K. (2012). Novel zeaxanthin-producing bacteria isolated from radioactive hot spring water. Methods in Molecular Biology, 892, 99–131.
Sajilata, M. G., Singhal, R. S., & Kamat, M. Y. (2008). The carotenoid pigment zeaxanthin a review. Comprehensive Reviews in Food Science and Food Safety, 7, 29–49.
Hadden, W. L., Watkins, R. H., Levy, L. W., Regalado, E., Rivadeneira, D. M., van Breemen, R. B., et al. (1999). Carotenoid composition of marigold (Tagetes erecta) flower extract used as nutritional supplement. Journal of Agricultural and Food Chemistry, 47, 4189–4194.
Gierhart, D. L. (1995). Zeaxanthin-containing compositions produced by Flavobacterium multivorum. US patent 5,427,783 (assignee, Applied Food Biotechnology Inc.; date of issue, June 27 1995.).
Bhosale, P., & Bernstein, P. S. (2004). Beta-carotene production by Flavobacterium multivorum in the presence of inorganic salts and urea. Journal of Industrial Microbiology and Biotechnology, 31, 565–571.
Asker, D., Beppu, T., & Ueda, K. (2007). Mesoflavibacter zeaxanthinifaciens gen. nov., sp. nov., a novel zeaxanthin-producing marine bacterium of the family Flavobacteriaceae. Systematic and Applied Microbiology, 30, 291–296.
Asker, D., Beppu, T., & Ueda, K. (2007). Zeaxanthinibacter enoshimensis gen. nov., sp. nov., a novel zeaxanthin-producing marine bacterium of the family Flavobacteriaceae, isolated from seawater off Enoshima Island, Japan. International Journal of Systematic and Evolutionary Microbiology, 57, 837–843.
Hameed, A., Arun, A. B., Ho, H. P., Chang, C. M., Rekha, P. D., Lee, M. R., et al. (2011). Supercritical carbon dioxide micronization of zeaxanthin from moderately thermophilic bacteria Muricauda lutaonensis CC-HSB-11T. Journal of Agriculture and Food Chemistry, 59, 4119–4124.
Hameed, A., Shahina, M., Lin, S. Y., Sridhar, K. R., Young, L. S., Lee, M. R., et al. (2012). Siansivirga zeaxanthinifaciens gen. nov., sp. nov., a novel zeaxanthin-producing member of the family Flavobacteriaceae isolated from coastal seawater of Taiwan. FEMS Microbiology Letters, 333, 37–45.
Arun, A. B., Chen, W. M., Lai, W. A., Chao, J. H., Rekha, P. D., Shen, F. T., et al. (2009). Muricauda lutaonensis sp. nov., a moderate thermophile isolated from a coastal hot spring. International Journal of Systematic and Evolutionary Microbiology, 59, 2738–2742.
Xu, R., Shang, N., & Li, P. (2011). In vitro and in vivo antioxidant activity of exopolysaccharide fractions from Bifidobacterium animalis RH. Anaerobe, 17, 226–231.
Gerhardt, P., Murray, R. G. E., Costilow, R. N., Nester, E. W., Wood, W. A., & Krieg, N. R. (1981). Manual of methods for general bacteriology. Washington: American Society for Microbiology.
Kämpfer, P., Dreyer, U., Neef, A., Dott, W., & Busse, H. J. (2003). Chryseobacterium defluvii sp. nov., isolated from wastewater. International Journal of Systematic and Evolutionary Microbiology, 53, 93–97.
Brosius, J., Palmer, M. L., Kennedy, P. J., & Noller, H. F. (1978). Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proceedings of the National Academy of Sciences, 75, 4801–4805.
Edwards, U., Rogall, T., Blöcker, H., Emde, M., & Böttger, E. C. (1989). Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Research, 17, 7843–7853.
Tamura, K., Dudley, J., Nei, M., & Kumar, S. (2007). MEGA 4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0. Molecular Biology and Evolution, 24, 1596–1599.
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., & Higgins, D. G. (1997). The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25, 4876–4882.
Felsenstein, J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution, 39, 783–791.
Zhang, E. X., & Yu, L. J. (1997). Studies on polysaccharide from Sargassum thunbergii for its ability to scavenge active oxygen species. Chinese Journal of Marine Drugs, 3, 1–4.
Nakagawa, T., & Yokozawa, T. (2002). Direct scavenging of nitric oxide and superoxide by green tea. Food and Chemical Toxicology, 40, 1745–1750.
Aquino, R., Morelli, S., Lauro, M. R., Abdo, S., Saija, A., & Tomaino, A. (2001). Phenolic constituents and antioxidant activity of an extract of Anthurium versicolor leaves. Journal of Natural Products, 64, 1019–1023.
Hwang, C. Y., Kim, M. H., Bae, G. D., Zhang, G. I., Kim, Y. H., & Cho, B. C. (2009). Muricauda olearia sp. nov., isolated from crude-oil-contaminated seawater, and emended description of the genus Muricauda. International Journal of Systematic and Evolutionary Microbiology, 59, 1856–1861.
Yoon, J. H., Lee, M. H., Oh, T. K., & Park, Y. H. (2005). Muricauda flavescens sp. nov. and Muricauda aquimarina sp. nov., isolated from a salt lake near Hwajinpo Beach of the East Sea in Korea, and emended description of the genus Muricauda. International Journal of Systematic and Evolutionary Microbiology, 55, 1015–1019.
Armstrong, G. A. (1997). Genetics of eubacterial carotenoid biosynthesis: A colorful tale. Annual Reviews of Microbiology, 51, 629–659.
Kirchman, D. L. (2002). The ecology of Cytophaga–Flavobacteria in aquatic environments. FEMS Microbiology Ecology, 39, 91–100.
Martinez, A., Bradley, A. S., Waldbauer, J. R., Summons, R. E., & DeLong, E. F. (2007). Proteorhodopsin photosystem gene expression enables photophosphorylation in a heterologous host. Proceedings of the National Academy of Sciences, 104, 5590–5595.
Sarada, R., Tripathi, U., & Ravishankar, G. A. (2002). Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process Biochemistry, 37, 623–627.
Thawornwiriyanun, P., Tanasupawat, S., Dechsakulwatana, C., Techkarnjanaruk, S., & Suntornsuk, W. Identification of newly zeaxanthin-producing bacteria isolated from sponges in the gulf of Thailand and their zeaxanthin production. Applied Biochemistry and Biotechnology, 167, 2357–2368.
Firdous, A. P., Preethi, K. C., & Kuttan, R. (2010). Antioxidant potential of meso-zeaxanthin a semi synthetic carotenoid. Food Chemistry, 119, 1096–1101.
Krinsky, N. I., Landrum, J. T., & Bone, R. A. (2003). Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annual Reviews of Nutrition, 23, 171–201.
Osborne, N. N., Casson, R. J., Wood, J. P., Chidlow, G., Graham, M., & Melena, J. (2004). Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Progress in Retinal and Eye Research, 23, 91–147.
Acknowledgments
This research work was kindly supported by a grant from Board Research of Nuclear Science BRNS 28/34/2009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prabhu, S., PD, R., Young, CC. et al. Zeaxanthin Production by Novel Marine Isolates from Coastal sand of India and its Antioxidant Properties. Appl Biochem Biotechnol 171, 817–831 (2013). https://doi.org/10.1007/s12010-013-0397-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0397-6