Abstract
The mammalian matrix metalloproteinase-9 (MMP-9), which might play a role in ovulation, uterus remodeling, embryo development, and implantation in mammals, is one of the potential functional candidate genes for porcine reproductive traits. In this study, the entire genomic sequence of porcine MMP-9 (pMMP-9) gene was established; it contains 13 exons and 12 introns. Real-time PCR analysis revealed that pMMP-9 is highly expressed in the Minzhu uterus before puberty and decreases significantly after sexual maturity (p < 0.05). Two single-nucleotide polymorphisms (A3011G and T5079C) that can be detected by PCR restriction fragment length polymorphism (PCR-RFLP) were discovered and tested for statistical associations with litter size traits in a crossbred population (Line DIV) derived from Landrace, Large White, Chinese Tongcheng and/or Chinese Meishan pigs. For A3011G, the GG genotype was associated with a significantly higher (p < 0.05) number of live births than those recorded for AA sows and the additive effect was significant (p < 0.05). The T5079C marker is not associated with litter size in this population. Further studies are needed to confirm the results of this study.
Similar content being viewed by others
Introduction
The matrix metalloproteinase (MMP) family controls the aspects of reproductive function, and great efforts have been made to understand the involvement of MMP-9 [1]. MMP-9 might play an important role in the degradation of uterine stromal extracellular matrix (ECM) [2, 3], and it participates in ovulation, implantation, and embryonic development [1, 4–6]. Gene knockout experiments demonstrated that MMP-9 deficiency impairs reproduction [7–9]. Although MMP-9-deficient mice can develop normally and are fertile [7, 8], the litters of these mice are smaller and the percentage of infertile breeding pairs is increased [9]. It is likely that porcine MMP-9 (pMMP-9) plays a role in reproduction; porcine follicular fluid contains pMMP-9 activity that is highly increased after administration of human chorionic gonadotropin [10]. In addition, pMMP-9 is present in porcine corpora lutea at a high level during the early luteal phase, luteolysis, and pregnancy [11–13]. Hence, pMMP-9 is a functional candidate gene for reproductive traits.
We cloned and characterized pMMP-9, examined the expression profile, and identified mutations in the pMMP-9 sequence. We examined the statistical association in a crossbred population derived from Landrace, Large White, Chinese Tongcheng and/or Chinese Meishan pigs (Line DIV population) to evaluate the importance of single-nucleotide polymorphisms (SNPs) in the litter size selection.
Materials and Methods
Animals and Data Collection
The association analysis was done in a Line DIV population that included 210 sows from a synthetic line of Landrace, Large White, Tongcheng, or Meishan origin that were raised in the experimental pig station of Huazhong Agricultural University. During 2003–2006, the total number of piglets born (TNB) and the number of piglets born alive (NBA) were recorded for 611 litters in Line DIV sows. Genomic DNA was prepared from blood samples using the standard phenol/chloroform extraction procedure [14].
Fifteen healthy Minzhu sows, a Chinese autochthonous breed, were used for the expression profiling analysis of the pMMP-9 gene. All animals were raised at the Minzhu conservation farms in Heilongjiang Province, China, and were weaned at the age of 45 days. There were five stages of development, including two stages (1 and 2.5 months) before puberty and three stages after sexual maturity (4, 6, and 8 months), and three pigs were sampled at each stage. In order to examine the developmental expression pattern of the target gene in the uterus, all uterine tissue samples were collected in these five stages. For the spatial expression pattern of the pMMP-9 gene, seven different tissues (heart, liver, spleen, lung, kidney, ovary, and uterus) were collected from three 8-month-old pigs. All tissue samples were immediately frozen in liquid nitrogen and stored at −80 °C.
Isolation of the pMMP-9 Gene and Sequence Analysis
Compared to the available cDNA sequences of the human MMP-9 gene (GenBank NM_004994) and the mouse MMP-9 gene (GenBank NM_013599), the pMMP-9 sequence available in GenBank contains a single open reading frame and 3′-untranslated region (3′-UTR) sequence information. Primers P-1, P-2, P-3, P-4, P-5, and P-6 (Table 1) were designed using Primer Premier 5.0 to amplify all the introns of the pMMP-9 gene on the basis of the pMMP-9 cDNA sequences (GenBank NM_001038004) and the exon/intron organization of the human MMP-9 gene (http://www.ncbi.nlm.nih.gov/IEB/Research/Ostell/Spidey) using genomic DNA. Each PCR reaction was done in a 25-μL reaction mix containing 50 ng of porcine genomic DNA, 0.5 μM each primer, 0.2 mM dNTPs, 1.5 mM MgCl2, and 2.0 units of Taq DNA polymerase in reaction buffer (Promega, Madison, Wisconsin, USA). PCR was done in a PTC-200 thermal cycler (Bio-Rad, Richmond, CA, USA) under the following conditions: 4 min at 94 °C then 35 cycles of 30 s at 94 °C, 45 s at the annealing temperature (Table 1), 90 s at 72 °C, and then a final extension step for 8 min at 72 °C. The PCR products were purified by agarose gel electrophoresis, cloned into the pMD18-T vector (TaKaRa, Tokyo, Japan), and sequenced commercially (Sangon, Shanghai, China).
RT-PCR Analysis of the Expression Patterns of the pMMP-9 Gene
Expression of the pMMP-9 gene in seven tissues and five developmental stages in the Minzhu sows was analyzed by quantitative real-time PCR (qRT-PCR) using primer pairs Exp and β-actin (β-actin GenBank NW_003299651; Table 1). Total RNA was extracted from samples using TRIzol® reagent (Invitrogen, Carlsbad, CA, USA). Purified RNA was suspended in RNase-free water and quantified by measurement of absorbance at a wavelength of 260 nm (A 260). Samples (3 μg) of RNA from heart, liver, spleen, lung, kidney, ovary, and uterus of three 8-month-old Minzhu pigs were subjected to reverse transcription of cDNA with M-MLV reverse transcriptase (Promega, Madison, WI, USA) according to the manufacturer's instructions. Transcription reaction without reverse transcriptase was used to control for DNA contamination.
qRT-PCR was done in an ABI 7500 Real-Time PCR System (Applied Biosystems, USA) using SYBR green I detection. Each PCR in a 25-μL reaction mix contained SYBR® Premix Ex Taq TM (TaKaRa, Tokyo, Japan), Rox II, gene-specific primers and template cDNA. The two-step RT-PCR protocol used (1) an initial denaturation step for 10 s at 95 °C then (2) 40 cycles of 5 s at 95 °C, 30 s at 60 °C. The standard curves of the pMMP-9 and β-actin gene were acquired by amplification of a serially diluted cDNA (undiluted, 1/10, 1/100, 1/1,000, and 1/10,000) to calculate the PCR efficiency. PCR was done in triplicate for each sample, and gene expression levels were quantified relative to the expression of the β-actin gene using 7500 System SDS software V1.3.1 (Applied Biosystems, USA) by the comparative C T (2−ΔΔCT) value method, in which the β-actin gene was used as an internal control to correct for the differences in the quantity of mRNA.
SAS software (version 8.0; SAS Institute, Cary, NC, USA) was used for statistical analysis. Difference of gene expression between groups was evaluated by analysis of variance and then by a Tukey–Kramer multiple comparison test. p < 0.05 was taken to indicate statistically significant difference.
Polymorphism Identification and Genotype with PCR-Restriction Fragment Length Polymorphism
Although the primer pairs used above were designed to cover all the introns of the pMMP-9 gene, they were partially overlapped and covered the exonic region. In order to identify genetic polymorphic sites, the pMMP-9 gene was sequenced in three large white Meishan and Qingping sows using these primer pairs. All PCR products were purified and sequenced, and comparative sequence analysis revealed several SNPs in pMMP-9 among these pigs. A PCR-RFLP assay was designed to genotype two selected SNPs. Primer pairs P-MspI and P-SmaI (Table 1) were used to identify A3011G in exon 7 and T5079C in intron 10 of the pMMP-9 gene, respectively. For the PCR-RFLP profile, 8.5 μL of PCR products amplified by primer P-MspI or P-SmaI was digested with 5 U of MspI or SmaI (TaKaRa, Tokyo, Japan) for 4 h at 37 °C and then separated by electrophoresis in an 8 % polyacrylamide gel in TBE buffer and stained with silver or on a 1.5 % agarose gel (with ethidium bromide) in TAE buffer.
Statistical Analysis
The effects of single genotypes on the traits studied were analyzed by the least squares method as applied in the general linear model procedure of SAS version 8.0 according to the following statistical model:
where Y ijklm is the observed value of a given trait, μ is the overall mean, P i is the effect of parity (i = 1, 2, 3, 4), Y j is the effect of year j (j = 1, 2, 3, 4), S k is the effect of season (k = 1, 2, 3, 4), L l is the effect of line l (l = 1, 2), G m is the effect of genotype m (m = 1, 2, 3), and e ijklm is the random residual.
In addition, the data were analyzed separately for first parity, later parity, and all parity. Both additive and dominance effects were estimated using the REG procedure of SAS version 8.0 according to the method of Liu [15], where the additive effect was estimated as −1, 0, and 1 for AA/TT, AG/TC, and GG/CC, respectively, and the dominance effect was represented as −1, 1, and −1 for AA/TT, AG/TC, and GG/CC, respectively.
Results
Isolation of the pMMP-9 Gene
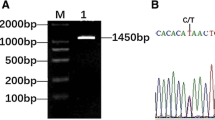
The 633, 1,311, 1,024, 1,360, 1,085, and 1,436 bp genomic fragments, which encompass all the introns, were amplified from total porcine genomic DNA using primer pairs 1–6, respectively (Fig. 1). The total mass of the published cDNA sequence for pMMP-9 (NM_001038004) is 2,302 bp which contains an open-reading frame of 2,127 nucleotides (nt) encoding a protein of 708 amino acids and the putative pMMP-9 (NP_001033093) contains one putative peptidoglycan binding domain, one zinc-dependent metalloprotease, and two Fibronectin Type II domain (http://www.ncbi.nlm.nih.gov/BLAST, Conserved Domain Architecture Retrieval Tool). Together with this cDNA sequence, the products described above were assembled into the genomic sequence of the pMMP-9 gene and deposited into GenBank with accession number DQ846655.
Amplification of pMMP-9 genomic sequence by PCR. a The PCR product amplified by primer pairs 1. b The PCR product amplified by primer pairs 2. c The PCR product amplified by primer pairs 3. d The PCR product amplified by primer pairs 4. e The PCR product amplified by primer pairs 5. f The PCR product amplified by primer pairs 6. The length of marker fragments is 2,000, 1,000, 750, 500, 250, and 100 bp
The putative exon and intron regions were identified by alignment of the pMMP-9 cDNA sequence with our genomic DNA sequence using Splign software (www.ncbi.nlm.nih.gov/sutils/splign; Fig. 2). The results revealed that the genomic sequence contains the entire 7,084 bp pMMP-9 gene, including 13 exons and 12 introns. Further comparison revealed that each of the 5′-donor and 3′-acceptor splice sites conformed to the expected consensus sequence for eukaryotic splice junctions, namely, the GT–AG rule.
Spatial and Temporal Expression Pattern of the pMMP-9 Gene
As shown in Fig. 3, the level of mRNA expression differed between tissues: high levels in heart, lung, and ovary; moderate levels in liver and spleen; and low levels in kidney and uterus. Figure 4 shows the expression patterns of pMMP-9 in different developmental stages of the uterus in Minzhu sows. The expression of pMMP-9 was increased drastically from 1–2.5 months and reached a peak at 2.5 months (p < 0.05). Then, it was decreased significantly and fell to the lowest level at 4 months (p < 0.05). pMMP-9 expression in the uterus remained stable after sexual maturity.
pMMP-9 mRNA expression during uterus development was analyzed by real-time PCR. The expression values were normalized with β-actin as standards, and the value of pMMP-9 in uterus of 4-month-old sows was arbitrarily set to 1. Expression refers to the mean ± SD. Different letters above each bar indicate significant difference at p < 0.05
Polymorphisms of the pMMP-9 Gene
Comparative sequence analysis revealed 12 polymorphic sites among the pMMP-9 genomic sequence in three pig breeds. These polymorphic sites consist of one synonymous A3011G in exon 7, and the other sites are widely distributed in introns. A3011G in exon 7 and T5079C in intron 10 can be detected by MspI and SmaI PCR-RFLP, respectively, so these SNPs were selected for further association analysis.
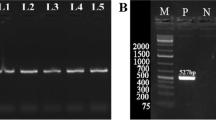
For the PCR-MspI-RFLP assay, the 307-bp PCR product was digested into two fragments of the A allele (192 and 105 bp) and three fragments of the G allele (192, 68, and 47 bp) for an additional MspI locus (Fig. 5). For the PCR-SmaI-RFLP, Fig. 6 shows that digestion of the 559-bp PCR product yielded the 559-bp fragment (the T allele) and 464 and 95 bp fragments of the C allele.
Association Between the pMMP-9 Genotype and Litter Size
Association analysis of two SNPs with different litter size traits indicated that the SmaI locus was not associated with the reproductive trait (data not given); however, the MspI locus was associated with NBA to a different extent (Table 2). Statistical analysis showed that there was no substantial difference among PCR-MspI-RFLP genotypes with litter size for the first parity, whereas for the latter litters, GG sows produced 1.08-fold greater (p < 0.05) NBA than the AA genotypes, and the additive effect was 0.54 piglet/litter (p < 0.05); for all parities, sows with a GG genotype produced 0.93-fold more (p < 0.05) NBA than AA genotypes, and the additive effect was 0.47 (p < 0.05). Table 2 shows that GG animals with the MspI locus appear to be associated with increased NBA.
Discussion
In this study, the pMMP-9 gene was cloned and expressed in all of the samples, including ovary and uterus. Then, real-time PCR analysis was employed to determine the mRNA expression pattern of pMMP-9 during Minzhu uterus development stages. The Minzhu was a prolific breed which came from far northern China, though not as prolific as Meishan and Erhualian breeds. They reach puberty at 3–4 months of age, achieve high embryo survival rates, and a large litter rate of 15–16 pigs (http://139.78.104.1/breeds/swine/). Recent research showed that the expression level of the MMP-9 gene in cows is stably maintained in the endometrium and fetal tissues throughout gestation [16]. In this study, we found that the expression of the pMMP-9 gene did not alter after puberty. So, in the future, expression patterns of this gene in ovary and endometrium during the porcine estrous cycle and/or gestation are needed for further understanding of the effect of this gene on reproductive processes.
In pigs, convincing evidence had been presented to support the hypothesis that litter size was limited by both ovulation rate and uterus ability. From these points, genes which encode special protein or hormone participated in the process of ovulation and/or implantation were selected as functional gene for reproduction traits. In mammalian MMP-9, which can degrade a variety of ECM components, was considered to be associated with remodeling ovary and uterus tissues in reproductive processes [1, 4, 5]. Hence, in this study, pMMP-9 was selected as a functional candidate gene for porcine reproductive traits.
In human, many polymorphisms (C-1562T, (CA)n) in MMP-9 gene were observed, and some were associated with the susceptibility to gestational hypertension [17]. In this study, firstly, we obtained the entire genomic sequence of pMMP-9 but observed only two SNPs that can be detected by PCR-RFLP. Association analysis showed that homozygous GG pigs had higher NBA than homozygous AA genotypes (p < 0.05) in later and in all parities, with a significant additive effect (p < 0.05). So, A3011G might be a potential marker for litter size, even if it did not alter the amino acid residues of the pMMP-9 gene, suggesting that this mutation affects litter size indirectly, being in linkage disequilibrium with a quantitative trait locus (QTL) or another causative polymorphism affecting reproduction traits. Interestingly, the pMMP-9 gene was located at 72.6 cM on SSC17 [18]; to the best of our knowledge, there is only one QTL for NBA at 22 cM on SSC17 [19]. Considering there is no relationship between litter size, QTL location, or the pMMP-9 position, this gene might be a linked marker but not the major causative mutation for this QTL. Of course, it is possible that the association result obtained was that this SNP might affect reproductive traits directly by regulating the expression of the pMMP-9 gene. However, this hypothesis needs to be confirmed by further research.
The data presented here suggested that the G allele in the pMMP-9 gene might confer a high prolificacy in breeding. However, the results of this study are based on limited populations with a limited number of animals and need to be confirmed in larger studies.
References
Chen, L., Nakai, M., Belton, R. J., Jr., & Nowak, R. A. (2007). Reproduction, 133, 405–414.
Collette, T., Bellehumeur, C., Kats, R., Maheux, R., Mailloux, J., Villeneuve, M., et al. (2004). Human Reproduction, 19, 1257–1264.
Nothnick, W. B. (2008). Seminars in Reproductive Medicine, 26, 94–99.
Lee, D. M., Lee, T. K., Song, H. B., & Kim, C. H. (2005). BJOG, 112, 946–951.
Daimon, E., & Wada, Y. (2005). Biology of Reproduction, 73, 163–171.
Bai, S. X., Wang, Y. L., Qin, L., Xiao, Z. J., Herva, R., & Piao, Y. S. (2005). Reproduction, 129, 103–113.
Vu, T. H., Shipley, J. M., Bergers, G., Berger, J. E., Helms, J. A., Hanahan, D., et al. (1998). Cell, 93, 411–422.
Itoh, T., Tanioka, M., Matsuda, H., Nishimoto, H., Yoshioka, T., Suzuki, R., et al. (1999). Clinical and Experimental Metastasis, 17, 177–181.
Dubois, B., Arnold, B., & Opdenakker, G. (2000). Journal of Clinical Investigation, 106, 627–628.
Driancourt, M. A., Quesnel, H., Meduri, G., Prunier, A., & Hermier, D. (1999). Journal of Reproduction and Fertility, 114, 287–297.
Pitzel, L., Ludemann, S., & Wuttke, W. (2000). Biology of Reproduction, 62, 1121–1127.
Ribeiro, L. A., Turba, M. E., Zannoni, A., Bacci, M. L., & Forni, M. (2006). BMC. Developmental Biology, 6, 58.
Ribeiro, L. A., Turba, M. E., Bernardini, C., Zannoni, A., Bacci, M. L., & Forni, M. (2007). Veterinary Research Communications, 1, 193–196.
Xiong, Y. Z. (1999). Pig biochemistry and molecular genetics: experimental introduction. Beijing: Chinese Agriculture Press.
Liu, B.H. (1998). Statistical Genomics: Linkage, Mapping and QTL Analysis. CRC Press, LLC
Kizaki, K., Ushizawa, K., Takahashi, T., Yamada, O., Todoroki, J., Sato, T., et al. (2008). Reproductive Biology and Endocrinology, 6, 66–78.
Palei, A. C., Sandrim, V. C., Duarte, G., Cavalli, R. C., Gerlach, R. F., & Tanus-Santos, J. E. (2010). Clinica Chimica Acta, 411, 874–887.
Ramos, A. M., Helm, J., Sherwood, J., Rocha, D., & Rothschild, M. F. (2006). Animal Genetics, 37, 296–297.
Noguera, J.L., Rodríguez M.C., Varona, L., Tomas, A., Munoz, G., Ramirez, O., Barragan, C., Arqué, M., Bidanel, J.P., Amills, M., Ovilo, C., & Sánchez, A. (2006). 8thWCGALP, Belo. Horizonte. (Brazil)
Acknowledgements
This study was supported financially by the Scientific Research Foundation of Northeast Agricultural University, China Postdoctoral Science Foundation (20080430875), and Heilongjiang Postdoctoral Science Foundation (LBH-Z08260).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niu, B., Li, F., Xiong, Y. et al. Characterization and Association Analysis with Litter Size Traits of Porcine Matrix Metalloproteinase-9 Gene (pMMP-9). Appl Biochem Biotechnol 171, 786–794 (2013). https://doi.org/10.1007/s12010-013-0396-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0396-7