Abstract
Genetic deficiency of acid alpha glucosidase (GAA) results in glycogen storage disease type II (GSDII) or Pompe’s disease. To investigate whether we could generate a functional recombinant human GAA enzyme (tobrhGAA) in tobacco seeds for future enzyme replacement therapy, we subcloned the human GAA cDNA into the plant expression plasmid-pBI101 under the control of the soybean β-conglycinin seed-specific promoter and biochemically analyzed the tobrhGAA. Tobacco seeds contain the metabolic machinery that is more compatible with mammalian glycosylation−phosphorylation and processing. We found the tobrhGAA to be enzymatically active was readily taken up by GSDII fibroblasts and in white blood cells from whole blood to reverse the defect. The tobrhGAA corrected the enzyme defect in tissues at 7 days after a single dose following intraperitoneal (IP) administration in GAA knockout (GAA−/−) mice. Additionally, we could purify the tobrhGAA since it bound tightly to the matrix of Sephadex G100 and can be eluted by competition with maltose. These data demonstrate indirectly that the tobrhGAA is fully functional, predominantly proteolytically cleaved and contains the minimal phosphorylation and mannose-6-phosphate residues essential for biological activity.
Similar content being viewed by others
Introduction
We investigated the potential of genetically engineered tobacco seeds as an alternative large-scale production system that would reduce the cost of producing recombinant human enzymes available to patients and at higher dosages than those currently administered. Several expression systems have been developed in plants that potentially offer many advantages in terms of production, scaling up, economy, and safety of the therapeutic molecules [1–4]. The technological platform involving the accumulation of recombinant proteins in seeds warrants a better availability of the products and allows long-term storage of the biomass for processing [5–7].
Transgenic plants, seeds, and cultured plant cells are potentially one of the most economical systems for large-scale production of recombinant enzymes for pharmaceutical uses [8–10]. Seeds are particularly attractive for use due to their high rates of protein synthesis and their ability to remain viable in the mature-dry state [3, 11, 12]. Over one third of approved pharmaceutical proteins are glycoproteins [13, 14] and even minor differences in N-glycan structures can change the distribution, activity, or longevity of recombinant proteins when compared with their native counterparts, altering their efficacy as therapeutics. Thus, one of the major challenges of using plants as systems for pharmaceutical glycoprotein production is to produce these pharmaceuticals with “humanized” N-glycans. Notably, certain processes of N-glycosylation that occur after proteins have left the post-endoplasmic reticulum (ER) along the secretory pathway are markedly different in plant cells versus mammalian cells. The early steps and components of the N-glycosylation process in the ER (including the involvement of the dolichol lipid intermediate and ER oligosaccharide transferase) and the Golgi-localized N-acetylglucosaminyl transferase I are the same in plant and mammalian cells [15]. For example, in the plant Golgi complex, enzymes convert the original high-mannose N-glycans of proteins to plant-specific hybrid and complex N-glycans by a series of sequential reactions that rely on the accessibility of the glycan chain(s) to the Golgi processing machinery [16, 17]. Plant-specific sugars that are associated with these “matured” N-glycans, such as β-1,2-xylose and α-1,3-fucose, may induce immune responses in humans, particularly when parenterally administrated [13, 18].
Acid alpha glucosidase (GAA) or acid maltase (EC 3.2.1.3) is a lysosomal enzyme that hydrolyzes glycogen to glucose [19]. The enzyme is apparently synthesized and processed via a pathway common to lysosomal enzymes [20, 21]. The native protein is initially synthesized as approximately 120 kD monomer and undergoes further trimming into two major bands of 80 and 70 kD and smaller-sized bands when analyzed on SDS-PAGE [22]. Genetic deficiency of GAA results in glycogen storage disease type II (GSDII) or acid maltase deficiency (AMD) (Pompe’s disease), encompassing at least five clinical subtypes of varying severity (infantile; non-classical infantile; childhood, juvenile, and late onset; [23]). The infantile form presents as hypotonia, muscle weakness, and congestive heart failure in the first year; the childhood and juvenile forms are fatal by the second decade of life, while the later onset forms are limited to skeletal muscle.
Currently, there is no effective treatment or cure for GSDII. Enzyme and gene replacement therapies are being developed. ERT by Genzyme, Inc. using a recombinant human GAA produced in a Chinese Hamster Ovary (CHO) cell line, has shown moderate success in patients using a biweekly infusion regimen. Thus, to provide a less expensive alternative, we wanted to evaluate a recombinant human GAA (tobrhGAA) produced in tobacco seeds for enzyme replacement therapy (ERT) of AMD for functional status by in vitro, ex vivo, and in vivo systems.
Material and Methods
RNA Extraction, cDNA Amplification, and Cloning
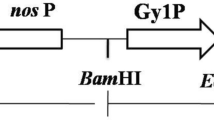
Total RNA was extracted from 200 mg of human placenta with TRIzol Reagent (Life Technologies) and poly(A)+ fraction isolated with the polyATract mRNA Isolation System (Promega) and reverse transcribed with M-MLV enzyme (Stratagene) using specific primers for the human GAA coding sequence (GAT ATC CTA ACA CCA GCT GAC GAG AAA CTG). Amplification of the GAA coding sequence was performed by combining the reverse primer with a second forward primer (GAT ATC TGC ACA CCC CGG CCG TCC CAG) matching the 5′ terminus of the cDNA sequence (GenBank acc. No. Y00839). An EcoRV site was inserted respectively in the forward and in the reverse primer to facilitate subsequent cDNA cloning in the plant expression vector. The cDNA for mature GAA was cloned under the control of the soybean β-conglycinin promoter (GenBank acc. No. M13759). The seed-specific promoter together with the relative 5′ UTR and transit peptide sequence was amplified from soybean DNA with primers inserting an XbaI and BamHI site (forward primer: TCT AGA GTT TTC AAA TTT GAA TTT TAA TGT GTG TTG and reverse primer: GGA TCC CAC CTT AAG GAG GTT GCA ACG AGC GTG GCA). Controlling elements and mature peptide sequence were assembled in pUC19 (Pharmacia-Amersham) and the whole tract cloned in pBI101 (Clontech) in place of the gusA gene.
Tobacco Transformation, Molecular Analysis of Transgenic Plants
The engineered plasmids were introduced in Agrobacterium tumefaciens strain EHA105 by electroporation. Tobacco leaf discs (Nicotiana tabacum L., cv.Xanthi) were transformed as described previously [24]. Putatively transformed (kanamycin-resistant) plants were potted in peat and hardened in a greenhouse together with controls (plants of the donor cultivar raised in vitro from uninfected discs). Total genomic DNAs were isolated from leaves of putative transgenic and wild-type tobacco plants as described by Doyle and Doyle [25] and evaluated by specific PCR. Genomic DNA of transgenic plants was extracted and PCR amplification to detect the GAA gene was carried out using primers specific for the human GAA coding sequence. Cycling conditions were: 94 °C × 2′; 94 °C × 45″; 58 °C × 45″;72 × 2′ for 40 cycles with a final 72 °C × 5′.
Protein Extraction
Seed samples (100 mg) were homogenized in mortar with pestle in the presence of 1 ml of extraction buffer (50 mM Tris, 5 mM EDTA, 200 mM NaCl, 0,1 % Tween 20, pH 8.0, and 10 mM PMSF). Samples were incubated on ice for 1 h under gentle agitation and eventually centrifuged at 14,000×g for 10 min. The supernatants were recovered and assayed for GAA using the artificial substrate 4-methylumbelliferyl-α-D-glucoside at pH 4.0 and as an internal control, neutral alpha glucosidase (NAG) was assayed at pH 7.5.
Western Analysis
Samples (80 μg total protein) were electrophoresed in a 10 % polyacrylamide gel and transferred to a nitrocellulose membrane (Hybond ECL, Amersham) with the Trans-Blot apparatus (BioRad) and filters were incubated for 1 h at room temperature with rabbit polyclonal anti-GAA serum (1:10,000; [26]). After incubation for 1 h with an HRP-conjugated secondary antibody (1:10,000), chemiluminescence was developed with ECL Western Blotting Detection Reagents (Amersham).
Enzyme Assay
GAA or NAG activity was determined using 100 μl of the artificial substrate 4-methylumbelliferyl-α-D-glucoside pH 4.0 for GAA or pH 7.5 for NAG for 2–24 h and fluorescence was determined in a fluorometer (excitation—360 nm and emission—460 nm; Sequoia-Turner) as previously described [27].
Purification of the tobrhGAA
Seeds were lysed as described above and clarified by centrifugation. The supernatant was adjusted to 1 mM EDTA, 25 mM sodium chloride pH 5.0 at 4 °C, and applied to a Sephadex G100 column (Amersham Pharmacia Biotech Inc.; [28]). The matrix was washed until no proteins were detected and the bound tobrhGAA was eluted in buffer containing 0.25 % maltose.
Uptake of tobrhGAA by GSD II Human Fibroblast Cells and Peripheral Blood Lymphocytes
Varying amounts of tobrhGAA (as crude extract equivalent to 1, 2, and 4 μg of tobrhGAA) were added to 106 SV40-Ad5 immortalized human GAA-deficient fibroblast cells (TR4912) in 10 % fetal bovine serum, DMEM (Life Technologies; [26]). Cells were harvested after various hours of exposure to the exogenous GAA, washed with PBS, lysed by sonication, and assayed for human GAA and NAG as described above.
Ex Vivo Experiments
We added a crude extract of tobrhGAA from 100 mg of seeds or placental GAA (4 μg) or mock-treated with PBS to 3 × 3 ml heparinized whole blood from an adult onset patient, incubated samples at 37 °C for 24 h on a rocker and WBCs were isolated with Accu-Prep (Accurate Chemical and Scientific Corp.). Cells were assayed for GAA and NAG.
In Vivo Studies in the GAA−/− Mice
We utilized the GAA−/− mouse with the exon 6neo disruption [29], wild-type BALB/c or GAA−/− mice mock-treated with PBS. We IP infused five GAA−/− mice (∼4 months old males) with a single dose of lysate from 300 mg (∼12 μg tobrhGAA) of transgenic seeds. At 7 days, mice were sacrificed and tissues were assayed for GAA and NAG and compared to wild-type mice and mock (PBS) treated GAA−/− mice.
Results
Our purpose was to accumulate the recombinant human GAA in the mature seed of tobacco, where recombinant proteins are stored in high quantity and stably maintain their enzymatic activity even after several months at room temperature. For promoter, we chose the gene coding for soybean β-conglycinin, a seed protein synthesized in very large. The expression of β-conglycinin is highly regulated, being restricted to the embryo during the mid-maturation phase of embryogenesis. The promoter sequence was amplified from genomic DNA of the soybean (Glycine max Merr.) and subcloned. To target the human GAA into the endoplasmic reticulum, the signal peptide sequence of soybean β-conglycin was used in place of the native signal to allow proper processing and translocation [6]. Therefore, promoter, 5′-UTR and shuttle peptide sequence were ligated upstream of the human GAA cDNA (Fig. 1). After mobilization of the engineered vector (pBI101-CONG-GAA) to A. tumefaciens EHA105, tobacco (N. tabacum, cv. Xanthi), transformation was carried out according to standard procedures [24]; several shoots (40 independently-transformed plants) survived levels of kanamycin (selective agent) as high as 100 μg/l. Molecular analysis confirmed the integration of the transgene in 87 % of transgenic plants. Western analysis demonstrated the tissue-specific expression of recombinant human GAA and its accumulation in developing seeds in 66 % of PCR-positive plants. The antibody reacted with two major bands of ∼80 and 70 kD, having an apparent molecular weight very similar to human placenta. No cross-reacting proteins were identified in wild-type seed extracts nor traces of degradation products in any transformed sample (data not shown).
Protein Extraction and Assay for GAA
One hundred milligrams of seeds from transgenic plants were homogenized and the supernatants assayed for GAA and as an internal control, neutral alpha maltase (NAG) was assayed at pH 7.5. Wild-type tobacco seeds had a GAA/NAG ratio of 0.05 while seeds from transgenic plants ranged from 0.1 to 2.0. Transgenic plant #3 had the greatest activity, estimated to contain 4 μg tobrhGAA/100 mg or 40 μg/g seeds. This extract was frozen and thawed 4× over 2 weeks without losing any substantial GAA activity.
Western Analysis
Extracts from seeds #3 were analyzed by Western analysis on a 10 % SDS-PAGE with rabbit polyclonal anti-placental GAA serum (data not shown). These data demonstrate that the tobrhGAA was similar to native human placental GAA showing two high molecular weight bands (∼80 and 70 kD) plus a third band of 100 kD. The 100-kD band may represent proteolytically uncleaved GAA. Smaller bands of 20–25 kD were not observed.
Uptake by GSDII Fibroblasts
A critical experiment to evaluate the functional status of the tobrhGAA is uptake by human GSDII fibroblast cells. Varying amounts of crude extract of seeds (equivalent to 1, 2, and 4 μg tobrhGAA) or 2.5, 5, and 10 μg purified human placental GAA (positive control) were added to human GSDII fibroblast cells. At 6 h, we found that cells exposed to either source of GAA had increased activity which increased as the amount of GAA was increased (Fig. 2). We found that at maximum amounts of tobrhGAA, 40 % of normal GAA was observed. Finally, to evaluate the longevity of the internalized GAA, we exposed cells to a constant amount of placental GAA or tobrhGAA for 6 h. We then replaced the media lacking any exogenous enzyme and harvested cells after 24, 48, and 168 h. Exposure to either GAA sources showed activity identical for 6 and 24 h incubation (data not shown). Minimal uptake was observed when cells were pretreated with 5 mM mannose-6-phosphate (data not shown).
Graph of uptake of tobrhGAA and placental GAA by GSDII fibroblast cells (mean ± SD). Varying amounts of crude extract of seeds (equivalent to 1, 2, and 4 μg tobrhGAA) or 2.5, 5, and 10 μg purified human placental GAA (positive control) was added to 106 human GSDII fibroblast cells. At 6 h, cells exposed to either source of GAA had increased activity which increased as the amount of GAA was increased. We estimate that the internalized tobrhGAA reversed the enzymatic defect in the fibroblasts to approximately 40 % of normal GAA activity
Ex Vivo Studies
We added a crude extract of tobrhGAA seeds (100 mg or ∼4 μg tobrhGAA calculated from specific activity) or placental GAA (4 μg) or mock-treated (PBS) to white blood cells (WBCs) from whole blood from an adult onset patient. After incubation, isolated WBCs were assayed for GAA. PBS mock-treated WBCs had a relative GAA activity of 5 (mean ±1); WBCs treated with the tobrhGAA had a relative GAA activity of 24 (mean ±6) while WBCs treated with placental GAA had a relative GAA activity of 35 (mean ±7) (Fig. 3). Student’s t test comparison between mock versus tobrhGAA treated cells was p < 0.007; mock versus placental GAA was p < 0.0003 and p < 0.02 for tobrhGAA versus placental GAA.
Graph of uptake of tobrhGAA and placental GAA in WBCs from adult GSDII whole blood (mean ± SD). A crude extract of tobrhGAA seeds (100 mg or ∼4 μg tobrhGAA) or placental GAA (4 μg) or mock treated with PBS to 3 × 3 ml heparinized whole blood from an adult onset patient, incubated samples with rocking at 37 °C for 24 h, and isolated WBCs by hypaque-ficol density centrifugation. WBCs cells mock treated with PBS had a relative GAA activity of 5 (mean ±1); cells treated with the tobrhGAA had a relative GAA activity of 24 (mean ±6) while cells treated with placental GAA had a relative GAA activity of 35 (mean ±7). Student’s t test comparison between mock versus tobrhGAA treated cells was p < 0.007; mock versus placental GAA was p < 0.0003 and p < 0.02 for tobrhGAA versus placental GAA
Purification of the tobrhGAA
Sephadex G100 is a natural affinity matrix for the mature, fully processed, glycosylated GAA [28]. If the mature enzyme is not processed and glycosylated, binding to Sephadex G100 will be very weak. To determine if the tobrhGAA can bind to Sephadex G100 (important for future large scale purification), we homogenized seeds and applied the supernatant to a Sephadex G100 column. The matrix was washed until no proteins were detected by A280 and the bound tobrhGAA was eluted in buffer containing 0.25 % maltose (Fig. 4). The specific GAA activity of the bound and eluted tobrhGAA was 8,000 IU/g as compared to purified human placental GAA of 12,000–15,000 IU/g as determined by enzyme assay. Recovery was approximately 15 %.
In Vivo Studies in GAA−/− Mice
To evaluate if the tobrhGAA can reverse the enzyme defect in tissues, we administered a lysate from 300 mg (∼12 μg tobrhGAA) transgenic seeds intraperitoneally (IP) to five GAA−/− mice (exon 6neo). At day 7, mice were sacrificed and tissues were assayed for GAA and NAG and compared to wild-type and mock-treated GAA−/− mice (Table 1). We found substanstial increases in GAA activity in tissues, most notably in heart, skeletal muscle, and diaphragm from GAA−/− mice treated with the tobrhGAA compared to mice mock-treated with PBS (mean ± SD). These levels were between 10 % and 20 % of wild-type GAA activity in tissues.
Discussion
Currently, there is no effective treatment or cure for GSDII. Lysosomal enzymes (such as GAA) are targeted to the lysosome by a mannose-6-phosphate recognition sequence that is exposed by posttranslational modification in the Golgi that may be the mechanism that extracellular GAA can be recycled and targeted back to the lysosomes. This mechanism will potentially allow recombinant human GAA to be delivered to the cells or tissues and directed to the lysosome. However, some GAA may be taken up or recycled by endocytosis or a mannose-6-phosphate independent mechanism [30–33]. A number of biotechnology companies have tried to mass produce a recombinant human GAA (rhGAA). A European biotechnology company (Pharming) started Phase I/II trials with a recombinant human GAA secreted into rabbit milk [34]. Although promising, their rhGAA was not successful in treating patients. A US company (Genzyme) using a rhGAA secreted from a CHO cell line has demonstrated moderate success in patients [35]; however, yearly costs are very high. Thus, to provide a less expensive alternative, we initiated experiments to generate and evaluate a recombinant human GAA produced in tobacco seeds for enzyme replacement therapy of AMD. Tobacco seeds contain the metabolic machinery that is more compatible with mammalian glycosylation−phosphorylation and processing. There have been a number of enzymes or proteins produced in seeds including human collagen type α-1 in maize seeds [36], human lysosomal α-mannosidase (MAN2B1) in Nicotiana benthamiana leaves and seeds [37], Ascaris suum As14 protein and its fusion with cholera toxin B subunit in rice seeds [38], cholera toxin B subunit in transgenic rice endosperm [39], human CD14 in tobacco seeds [40], human lactoferrin in maize and tobacco [41], and maize (Zea mays)-derived bovine trypsin characterization for large-scale, commercial product from transgenic plants [42]. We found that the tobrhGAA was enzymatically active and was readily taken up by GSDII fibroblasts. In WBCs from whole blood, the tobrhGAA corrected the enzyme defect in tissues at 7 days after a single intraperitoneal (IP) administration in GAA−/− mice. Additionally, we could easily purify the tobrhGAA because it bound tightly to the matrix of Sephadex G100 and could be eluted by competition with maltose. These data demonstrate indirectly that the tobrhGAA is fully functional, proteolytically cleaved and contains the minimal phosphorylation and mannose-6-phosphate residues to maintain activity. Only the native, fully processed human GAA binds tightly to Sephadex G100. Data in Escherichia coli [43] and unpublished data from our laboratory in yeast have found that the recombinant human GAA from both systems (that may have altered glycosylation/processing) show substantially reduced GAA activity despite the GAA mRNA being highly expressed. Additionally, the purified tobrhGAA has high specific activity, similar to the native human placental GAA making it ideal for enzyme replacement therapy. Estimates on production and costs are: 200 flowers per plant, ∼1,300 seeds per flower, and 1,000 seeds weighs 0.1 grams, thus 26 g of seeds per plant. There are 24,000 plants per acre or 60,000 per hectare. The cost to maintain and harvest seeds are $5,000/ha or $2,000/acre. A hectare can produce ∼1,444 kg of seeds. Our data suggests that there is 40 μg tobrhGAA/gram seeds or 1 hectare can produce 58 g of purified tobrhGAA. Hence, the cost of seed production per patient would be $540/year for a 50 mg/kg biweekly dose (2.5× higher than Myozyme’s dose of 20 mg/kg) or 62.5 g per year excluding purification costs. Current cost for ERT by Genzyme’s Myozyme ranges from $250,000 to $650,000 per patient depending upon weight. Before Phase I/II trials are considered, future evaluation of the tobrhGAA will include more detailed in vivo experiments in GAA−/− mice to determine dose regimens to reverse the clinical presentation, evaluate and determine survival, pharmacokinetics, and toxicity. Additionally, large-scale purification using Sephadex G100 needs to be optimized and stability of the tobrhGAA determined. In future experiments, we would need to compare the action of tobrhGAA to that of the currently available enzymes, Myozyme and Lumizyme, in all the tests undertaken in this study in order to demonstrate that it is no less effective than the presently available enzymes. Once that has been undertaken, we will be able to proceed with further the necessary animal and human studies.
Abbreviations
- IP:
-
intraperitoneal
- AMD:
-
acid maltase deficiency
- GSDII:
-
glycogen storage disease type II
- exon 6 neo:
-
exon 6 neomycin resistent
- rhGAA:
-
recombinant GAA
- tobrhGAA:
-
recombinant GAA produced in tobacco seeds
- GAA:
-
acid maltase
- ERT:
-
enzyme replacement therapy
References
Fischer, R., Schillberg, S., Hellwig, S., Twyman, R. M., & Drossard, J. (2012). GMP issues for recombinant plant-derived pharmaceutical proteins. Biotechnology Advances, 30, 434–439.
Lico, C., Santi, L., Twyman, R. M., Pezzotti, M., & Avesani, L. (2012). The use of plants for the production of therapeutic human peptides. Plant Cell Reports, 31, 439–451.
Twyman, R. M., Stoger, E., Schillberg, S., Christou, P., & Fischer, R. (2003). Molecular farming in plants: host systems and expression technology. Trends in Biotechnology, 21, 570–578.
Twyman, R. M., Ramessar, K., Quemada, H., Capell, T., & Christou, P. (2009). Plant biotechnology: the importance of being accurate. Trends in Biotechnology, 27, 609–612.
Kusnadi, A. R., Evangelista, R. L., Hood, E. E., Howard, J. A., & Nikolov, Z. L. (1998). Processing of transgenic corn seed and its effect on the recovery of recombinant beta-glucuronidase. Biotechnology and Bioengineering, 60, 44–52.
Reggi, S., Marchetti, S., Patti, T., De Amicis, F., Cariati, R., Bembi, B., & Fogher, C. (2005). Recombinant human acid β-glucosidase stored in tobacco seed is stable, active and taken up by human fibroblasts. Plant Molecular Biology, 57, 101–113.
Stoeger, E., Vaquero, C., Torres, E., Sack, M., Nicholson, L., Drossard, J., Williams, S., Keen, D., Perrin, Y., Christou, P., & Fischer, R. (2000). Cereal crops as viable production and storage systems for pharmaceutical scFv antibodies. Plant Molecular Biology, 42, 583–590.
Kermode, A. R. (2006). Plants as factories for production of biopharmaceutical and bioindustrial proteins: lessons from cell biology. Canadian Journal of Botany, 84, 679–694.
Kermode, A. R. (2012). Seed Expression Systems for Molecular Farming. In A. Wang & S. Ma (Eds.), Molecular farming in plants: recent advances and future prospects (pp. 89–123). New York: Springer.
Lau, O. S., & Sun, S. S. M. (2009). Plant seeds as bioreactors for recombinant protein production. Biotechnology Advances, 27, 1015–1022.
Boothe, J., Nykiforuk, C., Shen, Y., Zaplachinski, S., Szarka, S., Kuhlman, P., Murray, E., Morck, D., & Moloney, M. M. (2010). Seed-based expression system for plant molecular farming. Plant Biotechnology Journal, 8, 588–606.
Stoger, E., & Ma, J. K. (2005). Sowing the seeds of success: Pharmaceutical proteins from plants. Current Opinion in Biotechnology, 16, 167–173.3.
Gomord, V., Fischette, A. C., Menu-Bouaouiche, L., Saint-Jore-Dupas, C., Plasson, C., Michaud, D., & Faye, L. (2010). Plant-specific glycosylation patterns in the context of therapeutic protein production. Plant Biotechnology Journal, 8, 564–587.
Saint-Jore-Dupas, C., Faye, L., & Gomord, V. (2007). From planta to pharma with glycosylation in the toolbox. Trends in Biotechnology, 25, 317–323.
Lerouge, P., Cabanes-Macheteau, M., Rayon, C., Fischette-Lainé, A. C., Gomord, V., & Faye, L. (1998). N-glycosylation of recombinant pharmaceutical glycoproteins produced in transgenic plants: towards an humanisation of plant N-glycans. Plant Molecular Biology, 38, 31–48.
Gomord, V., & Faye, L. (2004). Posttranslational modification of therapeutic proteins in plants. Current Opinion in Plant Biology, 7, 171–181.
Kermode, A. R. (1996). Mechanisms of intracellular protein transport and targeting. Critical Reviews in Plant Sciences, 15, 285–423.
He, X., Galpin, J. D., Tropak, M. B., Mahuran, D., Haselhorst, T., von Itzstein, M., Kolarich, D., Packer, N. H., Miao, Y., Jiang, L., Grabowski, G. A., Clarke, L. A., & Kermode, A. R. (2012). Production of active human glucocerebrosidase in seeds of Arabidopsis thaliana complex-glycan-deficient (cgl) plants. Glycobiology, 22, 492–503.
Hers, H. G. (1963). Alpha-glucosidase deficiency in generalized glycogen storage disease (Pompe's Disease). Biochemical Journal, 86, 11–16.
Kornfeld, S. (1986). Trafficking of lysosomal enzymes in normal and disease states. The Journal of Clinical Investigation, 77, 1–6.
Rosenfeld, M. G., Kreibich, G., Popov, D., Kato, K., & Sabatini, D. D. (1982). Biosynthesis of lysosomal hydrolases: their synthesis in bound polysomes and the role of co- and post-translational processing in determining their subcellular distribution. The Journal of Cell Biology, 93, 135–141.
Oude Elferink, R.P.J. (1985) Biosynthesis, transport and processing of lysosomal alpha glucosidase. PhD Thesis, University of Amsterdam.
Slonim, A., Bulone, L., Ritz, S., Goldberg, T., Chen, A., & Martiniuk, F. (2000). Identification of two subtypes of infantile acid maltase deficiency: Evaluation of twenty-two patients and review of the literature. Journal of Pediatrics, 137, 283–285.
Horsch, R. B., Fry, J. E., Hoffmann, N. L., Eichholtz, D., Roger, S. D., & Fraley, R. T. (1985). A simple and general method for transferring genes into plants. Science, 227, 1229–1231.
Doyle, J. J., & Doyle, J. L. (1997). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin, 19, 11–15.
Martiniuk, F., Honig, J., & Hirschhorn, R. (1984). Further studies of the structure of human placental acid alpha-glucosidase. Archives of Biochemistry and Biophysics, 231, 454–460.
Martiniuk, F., & Hirschhorn, R. (1981). Characterization of neutral isozymes of human alpha-glucosidase. Biochimica et Biophysica Acta, 658, 248–261.
Martiniuk, F., Chen, A., Donnabella, V., Arvanitopoulos, E., Slonim, A., Raben, N., Plotz, P., & Rom, W. N. (2000). Correction of glycogen storage disease type II by enzyme replacement with a recombinant human acid maltase produced by over-expression in a CHO DHFRneg cell line. Biochem Biophys Res Comm., 276, 917–923.
Raben, N., Nagaraju, K., Lee, E., Kessler, P., Byrne, B., Lee, L., LaMaurex, M., King, J., Sauer, B., & Plotz, P. (1998). Targeted disruption of the acid alpha glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. Journal of Biological Chemistry, 273, 19086–19092.
Bijvoet, A. G., Kroos, M. A., Pieper, F. R., Van der Vliet, M., De Boer, H. A., Van der Ploeg, A. T., Verbeet, M. P., & Reuser, A. J. (1998). Recombinant human acid alpha-glucosidase: high level production in mouse milk, biochemical characteristics, correction of enzyme deficiency in GSDII KO mice. Human Molecular Genetics, 1815–24.
Maga, J. A., Zhou, J., Kambampati, R., Peng, S., Wang, X., Bohnsack, R. N., Thomm, A., Golata, S., Tom, P., Dahms, N. M., Byrne, B. J., & Lebowitz, J. H. (2013). Glycosylation-independent lysosomal targeting of acid α-glucosidase enhances muscle glycogen clearance in Pompe mice. Journal of Biological Chemistry, 288, 1428–1438.
Van der Ploeg, A. T., Bolhuis, P. A., Wolterman, R. A., Visser, J. W., Loonen, M. C., Busch, H. F., & Reuser, A. J. (1988). Prospect for enzyme therapy in glycogenosis II variants: a study on cultured muscle cells. Journal of Neurology, 235, 392–396.
Van der Ploeg, A. T., Kroos, M. A., Willemsen, R., Brons, N. H., & Reuser, A. J. (1991). Intravenous administration of phosphorylated acid alpha-glucosidase leads to uptake of enzyme in heart and skeletal muscle of mice. The Journal of Clinical Investigation, 87, 513–518.
Van den Hout, J., Kamphoven, J., Winkel, L. P., Arts, W. F., De Klerk, J. B., Loonen, M. C., Vulto, A. G., Cromme-Dijkhuis, A., Weisglas-Kuperus, N., Hop, W., Van Hirtum, H., Van Diggelen, O. P., Boer, M., Kroos, M. A., Van Doorn, P. A., Van der Voort, E., Sibbles, B., Van Corven, E. J., Brakenhoff, J. P., Van Hove, J., Smeitink, J. A., de Jong, G., Reuser, A. J., & Van der Ploeg, A. T. (2004). Long-term intravenous treatment of Pompe disease with recombinant human α-glucosidase from milk. Pediatrics, 113, 448–457.
Kikuchi, T., Yang, H. W., Pennybacher, M., Ichihara, N., Mizutani, M., van Hove, J. L. K., & Chen, Y. T. (1998). Clinical and metabolic correction of Pompe disease by enzyme therapy in acid maltase-deficient quail. Journal of Clinical Laboratory, 101, 827–833.
Xu, X., Gan, Q., Clough, R. C., Pappu, K. M., Howard, J. A., Baez, J. A., & Wang, K. (2011). Hydroxylation of recombinant human collagen type I alpha 1 in transgenic maize co-expressed with a recombinant human prolyl 4-hydroxylase. BMC Biotechnology, 11, 69–80.
De Marchis, F., Balducci, C., Pompa, A., Riise Stensland, H. M., Guaragno, M., Pagiotti, R., Menghini, A. R., Persichetti, E., Beccari, T., & Bellucci, M. (2011). Human α-mannosidase produced in transgenic tobacco plants is processed in human α-mannosidosis cell lines. Plant Biotechnology Journal, 9, 1061–1073.
Nozoye, T., Takaiwa, F., Tsuji, N., Yamakawa, T., Arakawa, T., Hayashi, Y., & Matsumoto, Y. (2009). Production of Ascaris suum As14 protein and its fusion protein with cholera toxin B subunit in rice seeds. Journal of Veterinary Medical Science, 71, 995–1000.
Oszvald, M., Kang, T. J., Tomoskozi, S., Jenes, B., Kim, T. G., Cha, Y. S., Tamas, L., & Yang, M. S. (2008). Expression of cholera toxin B subunit in transgenic rice endosperm. Molecular Biotechnology, 40, 261–268.
Blais, D. R., & Altosaar, I. (2006). Human CD14 expressed in seeds of transgenic tobacco displays similar proteolytic resistance and bioactivity with its mammalian-produced counterpart. Transgenic Research, 15, 151–164.
Samyn-Petit, B., Wajda Dubos, J. P., Chirat, F., Coddeville, B., Demaizieres, G., Farrer, S., Slomianny, M. C., Theisen, M., & Delannoy, P. (2003). Comparative analysis of the site-specific N-glycosylation of human lactoferrin produced in maize and tobacco plants. European Journal of Biochemistry, 270, 3235–3242.
Woodard, S. L., Mayor, J. M., Bailey, M. R., Barker, D. K., Love, R. T., Lane, J. R., Delaney, D. E., McComas-Wagner, J. M., Mallubhotla, H. D., Hood, E. E., Dangott, L. J., Tichy, S. E., & Howard, J. A. (2003). Maize (Zea mays)-derived bovine trypsin: characterization of the first large-scale, commercial protein product from transgenic plants. Biotechnology and Applied Biochemistry, 38, 123–130.
Martiniuk, F., Tzall, S., & Chen, A. (1992). Recombinant human acid alpha-glucosidase generated in bacteria: antigenic, but enzymatically inactive. DNA and Cell Biology, 11, 701–706.
Acknowledgements
This research was supported in part by a grant UL1 TR000038 from the National Center for Advancing Translational Sciences, National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Reprints should be directed to Frank Martiniuk.
Rights and permissions
About this article
Cite this article
Martiniuk, F., Reggi, S., Tchou-Wong, KM. et al. Production of a Functional Human Acid Maltase in Tobacco Seeds: Biochemical Analysis, Uptake by Human GSDII Cells, and In Vivo Studies in GAA Knockout Mice. Appl Biochem Biotechnol 171, 916–926 (2013). https://doi.org/10.1007/s12010-013-0367-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0367-z