Abstract
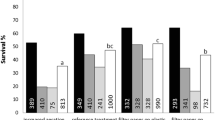
Subcultivation of Vero cells grown in a proprietary animal component-free medium named IPT-AFM, on microcarriers, was studied. TrypLE Select, a non-animal-derived protease, was used as an alternative to trypsin for cell passaging. We first studied the effect of increasing concentrations of TrypLE Select toward cell growth and then studied the inactivation of the protease using either soybean trypsin inhibitor (STI) or the soy hydrolysate Hypep 1510, in six-well plates. Data showed that cell growth was impaired by residual level of TrypLE Select; STI was identified as an efficient agent to neutralize this effect. To restore cell growth and inactivate TrypLE Select, STI should be added to the medium at least at 0.2 g L−1. Cells were also grown in spinner flask on 2 g L−1 Cytodex1 in IPT-AFM. In these conditions, the cell detachment yield was equal to 78 ± 8 %. Furthermore, cells exhibited a typical growth profile when using the dislodged cells to seed a new culture. A cell detachment yield of 70 ± 19 % was also achieved when the cells were grown in a 2-L stirred bioreactor in IPT-AFM, on 3 g L−1 Cytodex1. This protocol can be of great interest to scale-up the process of Vero cells cultivation in IPT-AFM on Cytodex1 from one stirred bioreactor culture to another.





Similar content being viewed by others
References
Birch, J. R. (1999). Suspension culture of animal cells. In M. C. Flickinger & S. W. Drew (Eds.), Encyclopedia of bioprocess technology: fermentation, biocatalysis, and bioseparation (pp. 2509–2516). New York: Wiley.
Shao, M., Lei, J., Wei, C., & Ouyang, F. (2002). Analysis of Vero cell growth behaviour on microcarriers by means of environmental scanning electron microscopy. Science in China (Series C), 45, 149–158.
Montagnon, B. J., Fanger, B., & Nicolas, A. J. (1981). The large-scale cultivation of Vero cells in microcarrier culture for virus vaccine production: preliminary results for killed poliovirus vaccine. Development in Biological Standardization, 47, 55–64.
Van Wezel, A. L. (1967). Growth of cell strains and primary cells on microcarriers in homogeneous culture. Nature, 216, 64–65.
Wang, Y., & Ouyang, F. (1999). Bead-to-bead transfer of Vero cells in microcarrier culture. Cytotechnology, 31, 221–224.
Lindner, E., Arvidsson, A. C., Wergeland, I., & Billig, D. (1987). Subpassaging cells on microcarriers: the importance for scaling up to production. Development in Biological Standardization, 66, 299–305.
Merten, O. W. (2000). Cell detachment. In M. C. Flickinger, S. W. Drew, & R. E. Spier (Eds.), The encyclopedia of industrial biotechnology: bioprocess, bioseparation and cell technology. New York: Wiley.
Merten, O. W. (2002). Development of serum-free media for cell growth and production of viruses/viral vaccines—safety issues of animal products used in serum-free media. Development in Biological Standardization, 111, 233–257.
Schröder, M., & Friedl, P. (1997). A protein-free solution as replacement for serum in trypsinization protocols for anchorage-dependent cells. Methods in Cell Science, 19, 137–147.
Lindskog, U., Lundgren, B., Billing, D., & Lindner, E. (1987). Alternatives for harvesting cells grown on microcarriers: effects on subsequent attachment and growth. Development in Biological Standardization, 66, 307–313.
Wiktor, T.J., Fanget, B.J., Montagnon, B.J. (1986). Process for the large scale production of rabies vaccine. US 4,664,912
Aert, B.L., Ghislain, Y.J.M., Gonze, M.M.J, Knott, ISL., Magetto, C. (2006) Animal free cell culture method. US Patent 0183224
Van Wezel, A. L., Van Der Velden-de Groot, C. A. T., & Van Herwaarden, J. A. (1980). The production of inactivated polio vaccine on serially cultivated kidney cells from captive-bred monkeys. Development in Biological Standardization, 46, 151–158.
Gebb, C., Lundgren, B., Clark, J., & Lindskog, U. (1984). Harvesting and subculturing cells growing on denatured-collagen coated microcarriers (Cytodex3). Development in Biological Standardization, 55, 57–65.
Billig, D., Clark, J. M., Ewell, A. J., Carte, C. M., et al. (1984). The separation of harvested cells from microcarriers: a comparison of methods. Development in Biological Standardization, 55, 67–75.
Reiter, M., Mundt, W., Grillberger, L., Kraus, B. (2004) Animal protein free media for cultivation of cells. WO Patent 005493A1.
Durocher, Y., Pham, P. L., St-Laurent, G., Jacob, D., et al. (2007). Scalable serum-free production of recombinant adeno associated virus type 2 by transfection of 293 suspension cells. Journal of Virological Methods, 144, 32–40.
Lenglois, S., Moser, M., & Miller, A. O. A. (2004). Microsupport with two-dimensional geometry (2D-MS). Cytotechnology, 44, 47–54.
Cinatl, J., Jr., Cinatl, J., Rabenau, H., Rapp, J., et al. (1993). Protein-free culture of Vero cells: a substrate for replication of human pathogenic viruses. Cell Biology International, 17, 885–896.
Cruz, H. J., Diaz, E. M., Moreira, J. L., & Carrondo, M. J. T. (1997). Cell dislodging methods under serum-free conditions. Applied Microbiology and Biotechnology, 47, 482–488.
Rourou, S., Van der Ark, A., Van der Velden, T., & Kallel, H. (2009). Development of an animal-component free medium for Vero cells culture. Biotechnology Progress, 25, 1752–1761.
Junge, L., Ohl, C. D., Wolfrum, B., Arora, M., et al. (2003). Cell detachment method using shock-wave-induced cavitation. Ultrasound in Medicine and Biology, 29, 1769–1776.
Brun-Graeppi, A. K. A. S., Richard, C., Bessodes, M., Scherman, D., et al. (2010). Thermoresponsive surfaces for cell culture and enzyme-free cell detachment. Progress in Polymer Science, 35, 1311–1324.
Yang, H. S., Jeon, O., Bhang, S. H., Lee, S. H., et al. (2010). Suspension culture of mammalian cells using thermosensitive microcarrier that allows cell detachment without proteolytic enzyme treatment. Cell Transplantation, 19, 1123–1132.
Brun-Graeppi, A. K., Richard, C., Bessodes, M., Scherman, D., et al. (2011). Cell microcarriers and microcapsules of stimuli-responsive polymers. Journal of Controlled Release, 149, 209–224.
Yoshikatsu, A., Akihiko, K., Masayuki, Y., & Teruo, O. (2004). Ultrathin poly (N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir, 20, 5506–5511.
Sun, X., & Zhang, Y. (2007). A cell-detaching reactor for inoculation of anchorage-dependent CHD and Vero cells between stepwise-expanded bioreactors. Biotechnology Letters, 29, 697–701.
Luo, F., Sun, H., Geng, T., & Qi, N. (2008). Application of Taguchi’s method in the optimization of bridging efficiency between confluent and fresh microcarriers in bead-to-bead transfer of Vero cells. Biotechnology Letters, 30, 645–649.
Rourou, S., Van der Ark, A., Van Der Velden, T., & Kallel, H. (2007). A microcarrier cell culture process for propagating rabies virus in Vero cells grown in a stirred bioreactor under fully animal component free conditions. Vaccine, 25, 3879–3889.
Rourou, S., Van der Ark, A., Majoul, S., Trabelsi, K., et al. (2009). A novel animal-component-free medium for rabies virus production in Vero cells grown on Cytodex 1 microcarriers in a stirred bioreactor. Applied Microbiology and Biotechnology, 85, 53–63.
Trabelsi, K., Rourou, S., Loukil, H., Majoul, S., et al. (2005). Comparison of various culture modes for the production of rabies virus by Vero cells grown on microcarriers in a 2-l bioreactor. Enzyme and Microbial Technology, 36, 517–519.
Umegaki, R., Kino-Oka, M., & Taya, M. (2004). Assessment of cell detachment and growth potential of human keratinocyte based on observed changes in individual cell area during trypsinization. Biochemical Engineering Journal, 17, 49–55.
Ozawa, K., & Laskowski, M. (1966). The reactive site of trypsin inhibitor. The Journal of Biological Chemistry, 241, 3955–3961.
Acknowledgments
We are grateful for Arno van der Ark and Tiny van der Velden from RIVM (Bilthoven, The Netherlands) for fruitful discussion. We also thank Sheffield-Bioscience for supplying the Hypeps.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rourou, S., Riahi, N., Majoul, S. et al. Development of an In Situ Detachment Protocol of Vero Cells Grown on Cytodex1 Microcarriers Under Animal Component-Free Conditions in Stirred Bioreactor. Appl Biochem Biotechnol 170, 1724–1737 (2013). https://doi.org/10.1007/s12010-013-0307-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0307-y