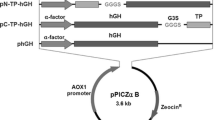
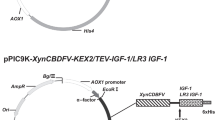
Abstract
Somatostatin is a natural inhibitor of growth hormone, and its analogues are clinically used for the therapy of acromegaly, gigantism, thyrotropinoma, and other carcinoid syndrome. However, natural somatostatin is limited for clinical usage because of its short half-life in vivo. Albumin fusion technology was used to construct long-acting fusion proteins, and Pichia pastoris was used as an expression system. Three fusion proteins, (somatostatin (SS)14)2-human serum albumin (HSA), (SS14)3-HSA, and HSA-(SS14)3, were constructed with different fusion copies of somatostatin-14 and fusion orientations. The expression level of (SS14)3-HSA and HSA-(SS14)3 was much lower than (SS14)2-HSA due to the additional fusion of the somatostatin-14 molecule. Matrix-assisted laser desorption ionization-time-of-flight mass spectrometry revealed that severe degradation occurred in the fermentation process. Similar to the standard of somatostatin-14, all three fusion proteins were able to inhibit growth hormone secretion in the blood, with (SS14)2-HSA being the most effective one. On the whole, (SS14)2-HSA was the most effective protein in both production level and bioactivity, and increasing the number of small protein copies fused to HSA may not be a suitable method to improve the protein bioactivity.





Similar content being viewed by others
References
Brazeau, P., Vale, W., Burgus, R., Ling, N., Butcher, M., Rivier, J., et al. (1973). Science, 179, 77–79.
Patel, Y. C. (1999). Frontiers in Neuroendocrinology, 20, 157–198.
Hofland, L. J. (2008). Molecular and Cellular Endocrinology, 286, 199–205.
Pyronnet, S., Bousquet, C., Najib, S., Azar, R., Laklai, H., & Susini, C. (2008). Molecular and Cellular Endocrinology, 286, 230–237.
Thermos, K., & Reisine, T. (1988). Molecular Pharmacology, 33, 370–377.
Olias, G., Viollet, C., Kusserow, H., Epelbaum, J., & Meyerhof, W. (2004). Journal of Neurochemistry, 89, 1057–1091.
Schonbrunn, A. (2008). Molecular and Cellular Endocrinology, 286, 35–39.
Patel, Y. C., & Wheatley, T. (1983). Endocrinology, 112, 220–225.
Lesche, S., Lehmann, D., Nagel, F., Schmid, H. A., & Schulz, S. (2009). Journal of Clinical Endocrinology and Metabolism, 94, 654–661.
van der Hoek, J., van der Lelij, A. J., Feelders, R. A., de Herder, W. W., Uitterlinden, P., Poon, K. W., et al. (2005). Clinical Endocrinology, 63, 176–184.
Olgac, V., Erbil, Y., Barbaros, U., Oztezcan, S., Giris, M., Kaya, H., et al. (2006). Digestive Diseases and Sciences, 51, 227–232.
Dou, W. F., Lei, J. Y., Zhang, L. F., Xu, Z. H., Chen, Y., & Jin, J. (2008). Protein Expression and Purification, 61, 45–49.
Halpern, W., Riccobene, T. A., Agostini, H., Baker, K., Stolow, D., Gu, M. L., et al. (2002). Pharmaceutical Research, 19, 1720–1729.
Osborn, B. L., Olsen, H. S., Nardelli, B., Murray, J. H., Zhou, J. X., Garcia, A., et al. (2002). Journal of Pharmacol and Experimental Therapeutics, 303, 540–548.
Gao, Z., Bai, G., Chen, J., Zhang, Q., Pan, P., Bai, F., et al. (2009). Bioscience, Biotechnology, and Biochemistry, 73, 688–694.
Zhan, J., Chen, Y., Yuan, H. Y., Li, H. and Lu, H. Biotechnology Letters, 34, 417–423.
Zhao, H. L., Xue, C., Wang, Y., Li, X. Y., Xiong, X. H., Yao, X. Q., et al. (2007). Journal of Biotechnology, 131, 245–252.
Acknowledgments
The authors thank Dr. Jian Jin and his colleagues for their technical assistance in fermentation and purification processes. The work is financially supported by the Natural Science Foundation of Jiangsu Province (BK2012104) and the public service platform for science and technology infrastructure construction project of Jiangsu Province (BM2012066).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, Y., Fan, J., Li, W. et al. The Effect of Albumin Fusion Patterns on the Production and Bioactivity of the Somatostatin-14 Fusion Protein in Pichia pastoris . Appl Biochem Biotechnol 170, 1637–1648 (2013). https://doi.org/10.1007/s12010-013-0304-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0304-1