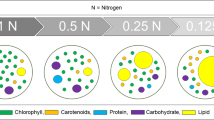
Abstract
Microalgae are one of the most promising biodiesel feedstocks due to their efficiency in CO2 fixation and high neutral lipid productivity. Nutrient–stress conditions, including nitrogen starvation, enhance neutral lipid content, but at the same time lead to a reduction of biomass. To maximize lipid production in the diatom Skeletonema marinoi, we investigated two different nitrogen starvation approaches. In the first experimental approach, inocula were effectuated in modified f/2 media with decreasing nitrogen concentration, while in the second experiment, nitrate concentration was gradually reduced through a collection/resuspension system in which the culture was periodically collected and resuspended in culture medium with a lower nitrate concentration. In the first approach, the neutral lipid accumulation was accompanied by a strong biomass reduction, as was expected, whereas the second experiment generated cultures with significantly higher neutral lipid content without affecting biomass production. The total proteins and total carbohydrates, which were also quantified in both experiments, suggest that in S. marinoi, neutral lipid accumulation during nutrient starvation did not derive from a new carbon partition of accumulated carbohydrates.






Similar content being viewed by others
References
Kim, S.-K., Ravichandran, Y. D., Khan, S. B., & Kim, Y. T. (2008). Biotechnology and Bioprocess Engineering, 13, 511–523.
Xu, L., Weathers, P. J., Xiong, X.-R., & Liu, C.-Z. (2009). Engineering in Life Sciences, 9, 178–189.
Sheehan, J., Dunahay, T., Benemann, J., & Roessler, P. (2008) A look back at the U.S. Department of Energy’s Aquatic Species Program: biodiesel from algae (NREL/TP-580-24190)
Pruvost, J., Van Vooren, G., Le Gouic, B., Couzinet-Mossion, A., & Legrand, J. (2011). Bioresource Technology, 102, 150–158.
Chisti, Y. (2008). Trends in Biotechnology, 26, 126–131.
Chisti, Y. (2007). Biotechnology Advances, 25, 294–306.
Lin, L., Cunshan, Z., Vittayapadung, S., Xiangqian, S., & Mingdong, D. (2011). Applied Energy, 88, 1020–1031.
Demirbas, A., & Fatih Demirbas, M. (2011). Energy Conversion and Management, 52, 163–170.
Mata, T. M., Martins, A. A., & Caetano, N. S. (2010). Renewable and Sustainable Energy Reviews, 14, 217–232.
Pittman, J. K., Dean, A. P., & Osundeko, O. (2011). Bioresource Technology, 102, 17–25.
Wang, B., Li, Y., Wu, N., & Lan, C. Q. (2008). Applied Microbiology and Biotechnology, 79, 707–718.
Scott, S. A., Davey, M. P., Dennis, J. S., Horst, I., Howe, C. J., Lea-Smith, D. J., et al. (2010). Current Opinion in Biotechnology, 21, 277–286.
Hsieh, C. H., & Wu, W. T. (2009). Bioresource Technology, 100, 3921–3926.
Yu, E. T., Zendejas, F. J., Lane, P. D., Gaucher, S., Simmons, B. A., & Lane, T. W. (2009). Journal of Applied Phycology, 21, 669–681.
Yeesang, C., & Cheirsilp, B. (2011). Bioresource Technology, 102, 3034–3040.
Pal, D., Khozin-Goldberg, I., Cohen, Z., & Boussiba, S. (2011). Applied Microbiology and Biotechnology, 90, 1429–1441.
Li, Y., Han, D., Sommerfeld, M., & Hu, Q. (2011). Bioresource Technology, 102, 123–129.
Hu, Q., Sommerfeld, M., Jarvis, E., Ghirardi, M., Posewitz, M., Seibert, M., et al. (2008). The Plant Journal, 54, 621–639.
Suen, Y., Hubbard, J. S., Holzer, G., & Tornabene, T. G. (1987). Journal of Phycology, 23, 289–296.
Roessler, P. G. (1988). Journal of Phycology, 24, 394–400.
Sarno, D., Kooistra, W. H. C. F., Medlin, L. K., Percopo, I., & Zingone, A. (2005). Journal of Phycology, 41, 151–176.
Leonardos, N., & Lucas, I. A. N. (2000). Aquaculture, 182, 301–315.
Brown, M. R., McCausland, M. A., & Kowalski, K. (1998). Aquaculture, 165, 281–293.
D'Souza, F. M. L., & Loneragan, N. R. (1999). Marine Biology, 133, 621–633.
Guillard, R. R. L. (1975). In M. H. Chanley & W. L. Smith (Eds.), Culture of marine invertebrate animals (pp. 26–60). New York: Plenum.
Guillard, R. R. L., & Ryther, J. H. (1962). Canadian Journal of Microbiology, 8, 229–239.
Bertozzini, E., Galluzzi, L., Penna, A., & Magnani, M. (2011). Journal of Microbiological Methods, 87, 17–23.
Sorokin, C. (1980). In J. R. Stein (Ed.), Handbook of phycological methods: culture methods and growth measurements (pp. 321–343). New York: Cambridge University Press.
González-Araya, R., Quéau, I., Quéré, C., Moal, J., & Robert, R. (2011). Aquaculture Research, 42, 710–726.
Peterson, G. L. (1983). Methods in Enzymology, 91, 95–121.
DuBois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Analytical Chemistry, 28, 350–356.
Parsons, T. R., Maita, Y., & Lalli, C. M. (1984). A manual of chemical and biological methods for seawater analysis (pp. 23–58). New York: Pergamon.
Li, Y., Horsman, M., Wang, B., Wu, N., & Lan, C. Q. (2008). Applied Microbiology and Biotechnology, 81, 629–636.
Breuer, G., Lamers, P. P., Martens, D. E., Draaisma, R. B., & Wijffels, R. H. (2012). Bioresource Technology, 124, 217–226.
Li, Y., Han, D., Hu, G., Dauvillee, D., Sommerfeld, M., Ball, S., et al. (2010). Metabolic Engineering, 12, 387–391.
Chiu, S. Y., Kao, C. Y., Tsai, M. T., Ong, S. C., Chen, C. H., & Lin, C. S. (2009). Bioresource Technology, 100, 833–838.
Mohammady, N. G. E., Rieken, C. W., Lindell, S. R., Reddy, C. M., Taha, H. M., Lau, C. P. L., et al. (2012). Research Journal of Phytochemistry, 6, 42–53.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(JPEG 54 kb)
High Resolution Image
(TIFF 501 kb)
Rights and permissions
About this article
Cite this article
Bertozzini, E., Galluzzi, L., Ricci, F. et al. Neutral Lipid Content and Biomass Production in Skeletonema marinoi (Bacillariophyceae) Culture in Response to Nitrate Limitation. Appl Biochem Biotechnol 170, 1624–1636 (2013). https://doi.org/10.1007/s12010-013-0290-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0290-3