Abstract
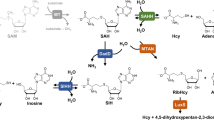
S-Adenosylhomocysteine hydrolase (SAHase) encoded by sahase gene is a determinant when catalyzing the reversible conversion of adenosine and homocysteine to S-adenosylhomocysteine in most living organisms. The sahase gene was isolated from the genome of the highly thermostable anaerobic bacteria Thermotoga maritima, and then it was cloned, characterized, overexpressed using Escherichia coli, and partially purified by thermal precipitation. The thermal purification of the recombinant SAHase resulted in changes in the circular dichroism spectra. As a result of this analysis, it was possible to determine the structural changes in the composition of the α-helix and β-sheet content of the recombinant enzyme after purification. Moreover, a predicted secondary structure and 3D structural model was rendered by comparative molecular modeling to further understand the molecular function of this protein including its attractive biotechnological use.






Similar content being viewed by others
Abbreviations
- SAHase:
-
S-Adenosyl-l-homocysteine hydrolase
- rSAHase:
-
Recombinant S-adenosyl-l-homocysteine hydrolase
- SAH:
-
S-Adenosyl-l-homocysteine
- ADO:
-
Adenosine
- Hcy:
-
Homocysteine
- Cys:
-
Cysteine
- CD:
-
Circular dichroism
- SAM:
-
S-Adenosylmethionine
- sahase :
-
S-Adenosylhomocysteine hydrolase coding gene
References
de la Haba, G., & Cantoni, G. L. (1959). J Parasit, 234, 603–608.
Ueland, P. M. (1982). Pharmacological Reviews, 34, 223–253.
Chiang, P. K., Gordon, R. K., Tal, J., Zeng, G. C., Doctor, B. P., Pardhasaradhi, K., et al. (1996). The FASEB Journal, 10, 471–480.
Yin, D., Yang, X., Hu, Y., Kuczera, K., Schowen, R. L., Borchardt, R. T., et al. (2000). Biochemistry, 39, 9811–9818.
Walker, R. D., & Duerre, J. A. (1975). Canadian Journal of Biochemistry, 53, 312–319.
Wnuk, S. F. (2001). Mini Rev. Medicinal Chemistry, 1, 307–316.
Kajander, E. O., & Raina, A. M. (1981). Biochemical Journal, 193, 503–512.
Henderson, D. M., Hanson, S., Allen, T., Wilson, K., Coulter-Karis, D. E., Greenberg, M. L., et al. (1992). Molecular and Biochemical Parasitology, 53, 169–183.
Creedon, K. A., Rathod, P. K., & Wellems, T. E. (1994). Journal of Biological Chemistry, 269, 16364–16370.
Cantoni, G. L. (1986). Biological methylation and drug design. In R. T. Borchardt, C. R. Creveiling, & P. M. Ueland (Eds.), The centrality of S-adenosylhomocysteinase in the regulation of the biological utilization of S-adenosylmethionine (pp. 227–238). Totowa: Humana.
Hayden, D. M., Rolletschek, H., Borisjuk, L., Corwin, J., Kliebenstein, D. J., Grimberg, A., et al. (2011). The Plant Journal, 67, 1018–1028.
Siu, K. K., Asmus, K., Zhang, A. N., Horvatin, C., Li, S., Liu, T., et al. (2011). Journal of Structural Biology, 173, 86–98.
Choi, J., Choi, D., Lee, S., Ryu, C. M., & Hwang, I. (2011). Trends in Plant Science, 16, 388–394.
Keller, W., & Bekkaoui, F. (2009). Botany, 87, 519–525.
Wu, X., Li, F., Kolenovsky, A., Caplan, A., Cui, Y., Cutler, A., et al. (2009). Botany, 87, 571–584.
Masuta, C., Tanaka, H., Uehara, K., Kuwata, S., Koiwai, A., & Noma, M. (1995). Proceedings of the National Academy of Sciences of the United States of America, 92, 6117–6121.
Hendricks, C. L., Ross, J. R., Pichersky, E., Noel, J. P., & Zhou, Z. S. (2004). Analytical Biochemistry, 326, 100–105.
Edwards, A. L., Reyes, F. E., Héroux, A., & Batey, R. T. (2010). RNA, 16, 2144–2155.
Collazo, E., Couture, J. F., Bulfer, S., & Trievel, R. C. (2005). Analytical Biochemistry, 342, 86–92.
Palmer, N. A., Sattler, S. E., Saathoff, A. J., & Sarath, G. (2010). Journal of Agricultural and Food Chemistry, 12, 5220–5226.
Bujnicki, J. M., Prigge, S. T., Cardinha, D., & Chiang, P. K. (2003). Proteins, 52, 624–632.
Kim, B. G., Chun, T. G., Lee, H. Y., & Snapper, M. L. (2009). Bioorganic & Medicinal Chemistry, 15, 6707–6714.
Carlucci, F., Tabucchi, A., Aiuti, A., Rosi, F., Floccari, F., Pagani, R., et al. (2003). Clinical Chemistry, 49, 1830–1838.
Baric, I., Fumic, K., Glenn, B., Cuk, M., Schulze, A., Finkelstein, J. D., et al. (2004). Proceedings of the National Academy of Sciences of the United States of America, 23, 4234–4239.
Liszka, M. J., Clark, M. E., Schneider, E., & Clark, D. S. (2012). Annual Review of Chemical and Biomolecular Engineering, 3, 77–102.
Porcelli, M., Fusco, S., Inizio, T., Zappia, V., & Cacciapuoti, G. (2000). Protein Expression and Purification, 18, 27–35.
Porcelli, M., Moretti, M. A., Concilio, L., Forte, S., Merlino, A., Graziano, G., et al. (2005). Proteins, 58, 815–825.
Marino, G., Nitti, G., Arnone, M. I., Sannia, G., Gambacorta, A., & De Rosa, M. (1998). Journal of Biological Chemistry, 263, 12305–12309.
Jones, C. E., Fleming, T. M., Cowan, D. A., Littlechild, J. A., & Piper, P. W. (1995). European Journal of Biochemistry, 233, 800–808.
Kumar, S., & Nussinov, R. (2001). Cellular and Molecular Life Sciences, 58, 1216–1233.
Fabry, S., & Hensel, R. (1987). European Journal of Biochemistry, 165, 147–155.
Simpson, H. D., Haufler, U. R., & Daniel, R. M. (1991). Biochemical Journal, 277, 413–417.
Koch, R., Canganella, F., Hippe, H., Jahnke, K. D., & Antranikian, G. (1997). Applied and Environmental Microbiology, 63, 1088–1094.
Klingeberg, M., Galunsky, B., Sjoholm, C., Kasche, V., & Antranikian, G. (1995). Applied and Environmental Microbiology, 61, 3094–3104.
Wassenberg, D., Liebl, W., & Jaenicke, R. (2000). Journal of Molecular Biology, 295, 279–288.
Andreotti, G., Cubellis, M. V., Nitti, G., Sannia, G., Mai, X., Adams, M. W. W., et al. (1995). Biochimica et Biophysica Acta, 1247, 90–96.
Vieille, C., & Zeikus, G. J. (2001). Microbiology and Molecular Biology Reviews, 65, 1–43.
Sreerama, N., Venyaminov, S. Y., & Woody, R. W. (2000). Analytical Biochemistry, 287, 243–251.
Bradford, M. M. (1976). Analytical Biochemistry, 72, 248–254.
Andrade, M. A., Chacón, P., Merelo, J. J., & Morán, F. (1993). Protein Engineering, 6, 383–390.
Merelo, J. J., Andrade, M. A., Prieto, A., & Morán, F. (1994). Neurocomputing, 6, 443–454.
Deleage, G., & Roux, B. (1987). Protein Engineering, 1, 289–294.
Geourjon, C., & Deleage, G. (1994). Protein Engineering, 7, 157–164.
Guermeur, Y., Geourjon, C., Gallinari, P., & Deleage, G. (1999). Bioinformatics, 15, 413–421.
King, R. D., & Sternberg, M. J. (1996). Protein Science, 5, 2298–2310.
Peitsch, M. C., Wells, T. N., Stampf, D. R., & Sussman, J. L. (1995). Trends in Biochemical Sciences, 20, 82–84.
Guex, N., & Peitsch, M. C. (1997). Electrophoresis, 18, 2714–2723.
Schwede, T., Kopp, J., Guex, N., & Peitsch, M. C. (2003). Nucleic Acids Research, 31, 3381–3385.
Lozada-Ramírez, J. D., Martínez-Martínez, I., Sánchez-Ferrer, A., & García-Carmona, F. (2006). Journal of Biochemical and Biophysical Methods, 67, 131–140.
Porcelli, M., Cacciapuoti, G., Fusco, S., Iacomino, G., Gambacorta, A., De Rosa, M., et al. (1993). Biochimica et Biophysica Acta, 1164, 179–188.
Huber, R., Langworthy, T. A., Köning, H., Thomm, M., Woese, C. R., Sleytr, U. B., et al. (1986). Archives of Microbiology, 144, 324–333.
Bouthier de la Tour, C., Portemer, C., Kaltoum, H., & Duguet, M. (1998). Journal of Bacteriology, 180, 274–281.
Yamada, T., Takata, Y., Komoto, J., Gomi, T., Ogawa, H., Fujioka, M., et al. (2005). International Journal of Biochemistry and Cell Biology, 37, 2417–2435.
Tanaka, N., Nakanishi, M., Kusakabe, Y., Shiraiwa, K., Yabe, S., Ito, Y., et al. (2004). Journal of Molecular Biology, 343, 1007–1017.
Bethin, K. E., Petrovic, N., & Ettinger, M. J. (1995). Journal of Biological Chemistry, 270, 20698.
Aguilar, C. F., Sanderson, I., Moracci, M., Ciaramella, M., Nucci, R., Rossi, M., et al. (1997). Journal of Molecular Biology, 271, 789–802.
Acknowledgments
This study was partially supported by Spanish grants from MINECO-FEDER (BIO2010-22225-C02-01) and from Programa de Ayuda a Grupos de Excelencia de la Región de Murcia, Fundación Séneca (04541/GERM/06, Plan Regional de Ciencia y Tecnología 2007–2010), and by Proyecto CONACYT Ciencia Básica 2009–2012 (CB-133949).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

Additional material 1
(A) 3D structural model of a single unit of SAHase from Thermotoga maritima. Alpha-helix are indicated in black, beta-strand in gray, and random coils in white. (B) Amino acid residues of the conserved domains implied in SAHase catalytic activity (Ado binding), and in NAD+ binding. Amino acid residues are discussed in the text. (JPEG 103 kb)

Additional material 2
Ribbon diagrams of the tetrameric structure of rat SAHase (A) and the theoretical structure of T. maritima SAHase. (B) Enlargements. (C) and (D) Amino acid residues which are involved in the network interactions in the central channel of rat SAHase and T. maritima SAHase, respectively. Amino acid residues are discussed in the text. (JPEG 175 kb)
Rights and permissions
About this article
Cite this article
Lozada-Ramírez, J.D., Sánchez-Ferrer, A. & García-Carmona, F. Recombinant S-Adenosylhomocysteine Hydrolase from Thermotoga maritima: Cloning, Overexpression, Characterization, and Thermal Purification Studies. Appl Biochem Biotechnol 170, 639–653 (2013). https://doi.org/10.1007/s12010-013-0218-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0218-y