Abstract
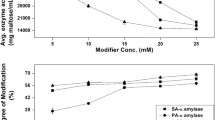
α-Amylase catalyzes hydrolysis of starch to oligosaccharides, which are further degraded to simple sugars. The enzyme has been widely used in food and textile industries and recently, in generation of renewable energy. An α-amylase from yeast Saccharomycopsis fibuligera R64 (Sfamy) is active at 50 °C and capable of degrading raw starch, making it attractive for the aforementioned applications. To improve its characteristics as well as to provide information for structural study ab initio, the enzyme was chemically modified by acid anhydrides (nonpolar groups), glyoxylic acid (GA) (polar group), dimethyl adipimidate (DMA) (cross-linking), and polyethylene glycol (PEG) (hydrophilization). Introduction of nonpolar groups increased enzyme stability up to 18 times, while modification by a cross-linking agent resulted in protection of the calcium ion, which is essential for enzyme activity and integrity. The hydrophilization with PEG resulted in protection against tryptic digestion. The chemical modification of Sfamy by various modifiers has thereby resulted in improvement of its characteristics and provided systematic information beneficial for structural study of the enzyme. An in silico structural study of the enzyme improved the interpretation of the results.




Similar content being viewed by others
Abbreviations
- Aotamy:
-
Aspergillus oryzae taka-amylase
- CC:
-
Cyanuric chloride
- DEAE:
-
Diethylaminoethyl
- DMA:
-
Dimethyl adipimidate
- DTT:
-
Dithiothreitol
- EDTA:
-
Ethylenediaminetetraacetate
- HIC:
-
Hydrophobic interaction chromatography
- PAGE:
-
Polyacrylamide gel electrophoresis
- PEG:
-
Polyethylene glycol
- PEGylation:
-
Modification with polyethylene glycol
- SDS:
-
Sodium dodecyl sulfate
- Sfamy:
-
Saccharomycopsis fibuligera α-amylase
- TIM:
-
Triosephosphate isomerase
- TNBS:
-
Trinitrobenzene sulfonate
- TPCK:
-
L-1-Tosylamido-2-phenylethyl chloromethyl ketone
References
Janecek, S., & Balaz, S. (1992). FEBS Letters, 304, 1–3.
van der Maarel, M. J. E. C., van der Veen, B., Uitdehaag, J. C. M., Leemhuis, H., & Dijkhuizen, L. (2002). Journal of Biotechnology, 94, 137–155.
Nielsen, J. E., & Borchert, T. V. (2000). Biochimica et Biophysica Acta, 1543, 253–274.
Shigechi, H., Koh, J., Fujita, Y., Matsumoto, T., Bito, Y., Ueda, M., et al. (2004). Applied and Environmental Microbiology, 70, 5037–5040.
McCue, P. P., & Shetty, K. (2004). Process Biochemistry, 39, 1785–1791.
Sidhu, G. S., Sharma, P., Chakrabarti, T., & Gupta, J. K. (1997). Enzyme and Microbial Technology, 21, 525–530.
Hasan, K., Ismaya, W. T., Kardi, I., Andiyana, Y., Kusumawidjaya, S., Ishmayana, S., et al. (2008). Biologia, 63, 1044–1050.
Ulmer, K. M. (1983). Science, 219, 666–671.
Janecek, S. (1993). Process Biochemistry, 28, 435–445.
Mozhaev, V. V., Siksnis, V. A., Melik-Nubarov, N. S., Galkantaite, N. Z., Denis, G. J., Butkus, E. P., et al. (1988). European Journal of Biochemistry, 173, 147–154.
Mozhaev, V. V., Melik-Nubarov, N. S., Siksnis, V. A., & Martinek, K. (1990). Biocatalyst, 3, 189–196.
Brzozowski, A. M., & Davies, G. J. (1997). Biochemistry, 36, 10837–10845.
Itoh, T., Yamashita, I., & Fukui, S. (1987). FEBS Letters, 29, 339–342.
Matsui, I., Yoneda, S., Ishikawa, K., Miyairi, S., Fukui, S., Umeyama, H., et al. (1994). Biochemistry, 33, 189–196.
Janecek, S., Svensson, B., & Henrissat, B. (1997). Journal of Molecular Evolution, 45, 322–331.
Janecek, S., & Sevcik, J. (1999). FEBS Letters, 456, 119–125.
Svensson, B. (1994). Plant Molecular Biology, 25, 141–157.
Franco, T. T., Andrews, A. T., & Ansejo, J. A. (1996). Biotechnology and Bioengineering, 49, 290–299.
Franco, T. T., Andrews, A. T., & Ansejo, J. A. (1996). Biotechnology and Bioengineering, 49, 300–308.
Alcalde, M., Plou, F. J., Anderssen, C., Martin, M. T., Pederssen, S., & Ballesteros, A. (1999). FEBS Letters, 445, 333–337.
Melik-Nubarov, N. S., Mozhaev, V. V., Siksnis, S., & Martinek, K. (1987). Biotechnology Letters, 9, 725–730.
Kazan, D., Ertan, H., & Erarslan, A. (1996). Process Biochemistry, 31, 135–140.
Takashi, K., Ajima, A., Takayuki, Y., Okada, M., Matsushima, A., Tamaura, Y., et al. (1985). Journal of Organic Chemistry, 50, 3411–3415.
Fuwa, H. (1954). J. Biochem (Tokyo), 41, 583–603.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Journal of Biological Chemistry, 193, 265–275.
Fields, R. (1971). Biochemical Journal, 124, 581–590.
Sadana, A., & Henley, J. P. (1986). Biotechnology and Bioengineering, 28, 256–268.
Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., McGettigan, P. A., McWilliam, H., et al. (2007). Bioinformatics, 23, 2947–2948.
Goujon, M., McWilliam, H., Li, W., Valentin, F., Squizzato, S., Paern, J., et al. (2010). Nucleic Acid Research, 38, W695–W699.
Arnold, K., Bordoli, L., Kopp, J., & Schwede, T. (2006). Bioinformatics, 22, 195–201.
Collaborative Computational Project Number 4. (1994). Acta Crystallographica, D50, 760–763.
Chen, V. B., Arendall, W. B., III, Headd, J. J., Keedy, D. A., Immormino, R. M., Kapral, G. J., et al. (2010). Acta Crystallographica, D66, 12–21.
Emsley, P., Lohkamp, B., Scott, W., & Cowtan, K. (2010). Acta Crystallographica, D66, 486–501.
DeLano, W. L. (2008) The PyMOL molecular graphics system, Delano Scientific LLC, Palo Alto, CA - USA.
Dolinsky, T. J., Nielsen, J. E., McCammon, J. A., & Baker, N. A. (2004). Nucleic Acid Research 32, W665–W667.
Baker, N. A., Sept, D., Joseph, S., Holst, M. J., & McCammon, J. A. (2001). Proceedings of the National Academy of Sciences, 98, 10037–10041.
Tsai, C. S., Tsai, Y. H., Lauzon, G., & Cheng, S. T. (1974). Biochemistry, 13, 440–443.
Hirs, C. H. W., & Kycia, J. H. (1965). Archives of Biochemistry and Biophysics, 111, 223–235.
Paetzel, M., Strynadka, N. C. J., Tschantz, W. R., Casareno, R., Bullinger, P. R., & Dalbey, R. E. (1997). Journal of Biological Chemistry, 272, 9994–10003.
Siddiqui, K. S., Poljak, A., Guilhaus, M., Francisci, D. D., Curmi, P. M. G., Feller, G., et al. (2006). Proteins, 64, 486–501.
Baines, N. J., Baird, J. B., & Elmore, D. T. (1964). Biochemical Journal, 90, 470–476.
Seidl, D. S., & Liener, I. E. (1971). Biochemical and Biophysical Research Communications, 42, 1101–1107.
Nureddin, A., & Inagami, T. (1975). Biochemical Journal, 147, 71–81.
Vieille, C., & Zeikus, J. G. (1996). Tibtech, 14, 183–189.
Fu, M.-X., Requena, J. S. R., Jenkins, A. J., Lyons, T. J., Baynes, J. W., & Thorpe, S. R. (1996). Journal of Biological Chemistry, 271, 9982–9986.
Kislinger, T., Fu, C., Huber, B., Qu, W., Taguchi, A., Du Yan, S., et al. (1999). Journal of Biological Chemistry, 274, 31740–31749.
Buetler, T. M., Leclerc, E., Baumeyer, A., Latado, H., Newell, J., Adolfsson, O., et al. (2008). Molecular Nutrition & Food Research, 52, 370–378.
Sinz, A. (2006). Mass Spectrometry Reviews, 25, 663–682.
Abuchowski, A., McCoy, J. R., Palczuk, N. C., van Es, T., & Davis, F. F. (1977). Journal of Biological Chemistry, 252, 3582–3586.
Matsushima, A., Nishimura, H., Ashihara, Y., Yokota, Y., & Inada, Y. (1980). Chemistry Letters, 773–776.
McGoff, P., Baziotis, A. C., & Maskiewicz, R. (1988). Chemical and Pharmaceutical Bulletin, 36, 3079–3091.
Leiros, H.-K. S., Brandsdal, B. O., Andersen, O. A., Os, V., Leiros, I., Helland, R., et al. (2004). Protein Science, 13, 1056–1070.
Sada, E., Katoh, S., Yamanaka, K., & Itakura, Y. (1991). Journal of Fermentation and Bioengineering, 71, 137–139.
Francis, G. E., Delgado, C., Fisher, D., Malik, F., & Agrawal, A. K. (1996). Journal of Drug Targeting, 3, 321–340.
Acknowledgments
The research was financed by the Indonesian Directorate General of Higher Education (DIKTI), Ministry of National Education, Republic of Indonesia (competitive grant batch IX/1-3 entitled Protein engineering of α-amylase from Saccharomycopsis fibuligera R64, for WTI). We thank Prof. J.J. Beintema for his valuable advice and discussion and Dr. H.J. Doddema for editing the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ismaya, W.T., Hasan, K., Kardi, I. et al. Chemical Modification of Saccharomycopsis fibuligera R64 α-Amylase to Improve its Stability Against Thermal, Chelator, and Proteolytic Inactivation. Appl Biochem Biotechnol 170, 44–57 (2013). https://doi.org/10.1007/s12010-013-0164-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0164-8