Abstract
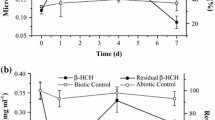
The organochlorine pesticide γ-hexachlorocyclohexane (γ-HCH, lindane) and its non-insecticidal isomers α-, β-, and δ- continue to pose serious environmental and health concerns, although their use has been restricted or completely banned for decades. The present study reports the first results on the ability of two Arthrobacter strains, not directly isolated from a HCH-polluted site, to grow in a mineral salt medium containing α-, β-, or γ-HCH (100 mg l−1) as sole source of carbon. Growth of cultures and HCHs degradation by Arthrobacter fluorescens and Arthrobacter giacomelloi were investigated after 1, 2, 3, 4, and 7 days of incubation by enumerating colony forming units and GC with ECD detection, respectively. Both bacteria are able to metabolize the HCHs: A. giacomelloi is the most effective one, as after 72 h of incubation it produces 88 % degradation of α-, 60 % of β-, and 56 % of γ-HCH. The formation of possible persistent compounds was studied by GC/MS and by HPLC analysis. Pentachlorocyclohexenes and tetrachlorocyclohexenes have been detected as metabolites, which are almost completely eliminated after 72 h of incubation, while no phenolic compounds were found.


Similar content being viewed by others
References
Vijgen, J., Abhilash, P. C., Li, Y. F., Lal, R., Forter, M., Torres, J., et al. (2011). Hexachlorocyclohexane (HCH) as new Stockholm Convention POPs—a global perspective on the management of Lindane and its waste isomers. Environmental Science and Pollution Research, 18, 152–162.
Willett, K. L., Ulrich, E. M., & Hites, R. A. (1998). Differential toxicity and environmental fates of hexachlorocyclohexane isomers. Environmental Science & Technology, 32, 2197–2207.
Srivastava, A., & Shivanandappa, T. (2010). Stereospecificity in the cytotoxic action of hexachlorocyclohexane isomers. Chemico-Biological Interactions, 183, 34–39.
Pavlíková, N., Bláhová, L., Klán, P., Reddy Bathula, S., Sklenář, V., Giesy, J. P., et al. (2012). Enantioselective effects of alpha-hexachlorocyclohexane (HCH) isomers on androgen receptor activity in vitro. Chemosphere, 86, 65–69.
Engst, R., Fritsche, W., Knoll, R., Kujawa, M., Macholz, R. M., & Straube, G. (1979). Interim result of studies of microbial isomerization of gamma-hexachlorocyclohexane. Bulletin of Environmental Contamination and Toxicology, 22, 699–707.
Buser, H. R., & Muller, M. D. (1995). Isomer and enantioselective degradation of hexachlorocyclohexane isomers in sewage sludge under anaerobic conditions. Environmental Science & Technology, 29, 664–672.
Phillips, T. M., Seech, A. G., Lee, H., & Trevors, J. T. (2005). Biodegradation of hexachlorocyclohexane (HCH) by microorganisms. Biodegradation, 16, 363–392.
Senoo, K., & Wada, H. (1989). Isolation and identification of an aerobic γ-HCH decomposing bacterium from soil. Soil Science and Plant Nutrition, 35, 79–87.
Sahu, S. K., Patnaik, K. K., Sharmila, M., & Sethunathan, N. (1990). Degradation of alpha-, beta-, and gamma-hexachlorocyclohexane by a soil bacterium under aerobic conditions. Applied and Environmental Microbiology, 56, 3620–3622.
Johri, A. K., Dua, M., Tuteja, D., Saxena, R., Saxena, D. M., & Lal, R. (1998). Degradation of alpha, beta, gamma and delta-hexachlorocyclohexane by Sphingomonas paucimobilis. Biotechnology Letters, 20, 885–887.
Datta, J., Maiti, A. K., Modak, D. P., Chakrabartty, P. K., Bhattacharyya, P., & Ray, P. K. (2000). Metabolism of γ-hexachlorocyclohexane by Arthrobacter citreus strain BI-100: identification of metabolites. Journal of General and Applied Microbiology, 46, 59–67.
Gupta, A., Kaushik, C. P., & Kaushik, A. (2000). Degradation of hexachlorocyclohexane (HCH; α, β, γ and δ) by Bacillus circulans and Bacillus brevis isolated from soil contaminated with HCH. Soil Biology and Biochemistry, 32, 1803–1805.
Manickam, N., Mau, M., & Schlömann, M. (2006). Characterization of the novel HCH-degrading strain Microbacterium sp. ITRC1. Applied Microbiology and Biotechnology, 69, 580–588.
Manickam, N., Reddy, M. K., Saini, H. S. & Shanker, R. (2008). Isolation of hexachlorocyclohexane-degrading Sphingomonas sp. by dehalogenase assay and characterization of genes involved in γ-HCH degradation. Journal of Applied Microbiology, 104, 952–960.
Kaur, J., Verma, M. & Lal, R. (2011). Rhizobium rosettiformans sp. nov., isolated from a hexachlorocyclohexane dump site, and reclassification of Blastobacter aggregatus Hirsch and Müller 1986 as Rhizobium aggregatum comb. nov. International Journal of Systematic and Evolutionary Microbiology, 61, 1218–1225.
Pesce, S. F., & Wunderlin, D. A. (2004). Biodegradation of lindane by a native bacterial consortium isolated from contaminated river sediment. International Biodeterioration & Biodegradation, 54, 255–260.
van Doesburg, W., van Eekert, M. H., Middeldorp, P. J., Balk, M., Schraa, G., & Stams, A. J. (2005). Reductive dechlorination of beta-hexachlorocyclohexane (beta-HCH) by a Dehalobacter species in coculture with a Sedimentibacter sp. FEMS Microbiology Ecology, 54, 87–95.
Elcey, C. D., & Kunhi, A. A. M. (2010). Substantially enhanced degradation of hexachlorocyclohexane isomers by a microbial consortium on acclimation. Journal of Agricultural and Food Chemistry, 58, 1046–1054.
Nagata, Y., Endo, R., Ito, M., Ohtsubo, Y., & Tsuda, M. (2007). Aerobic degradation of lindane (γ-hexachlorocyclohexane) in bacteria and its biochemical and molecular basis. Applied Microbiology and Biotechnology, 76, 741–752.
Jagnow, G., Haider, K., & Ellwardt, P. C. (1977). Anaerobic dechlorination and degradation of hexachlorocyclohexane isomers by anaerobic and facultative anaerobic bacteria. Archives of Microbiology, 115, 285–292.
Lal, R., Dadhwal, M., Kumari, K., Sharma, P., Singh, A., Kumari, H., Jit, S., Gupta, S. K., Nigam, A., Lal, D., Verma, M., Kaur, J., Bala, K. & Jindal, S. (2008). Pseudomonas sp. to Sphingobium indicum: a journey of microbial degradation and bioremediation of hexachlorocyclohexane. Indian Journal of Microbiology, 48, 3–18.
Camacho-Pérez, B., Ríos-Leal, E., Rinderknecht-Seijas, N., & Poggi-Varaldo, H. M. (2011). Enzymes involved in the biodegradation of hexachlorocyclohexane: a mini review. Journal of Environmental Management. doi:10.1016/j.jenvman.2011.06.047.
Raina, V., Rentsch, D., Geiger, T., Sharma, P., Buser, H. R., Holliger, C., et al. (2008). New metabolites in the degradation of α- and γ-hexachlorocyclohexane (HCH): pentachlorocyclohexenes are hydroxylated to cyclohexenols and cyclohexenediols by the haloalkane dehalogenase LinB from Sphingobium indicum B90A. Journal of Agricultural and Food Chemistry, 56, 6594–6603.
Zheng, G., Selvam, A., & Wong, J. W. C. (2011). Rapid degradation of lindane (γ-hexachlorocyclohexane) at low temperature by Sphingobium strains. International Biodeterioration & Biodegradation, 65, 612–618.
Nagata, Y., Miyauchi, K., & Takagi, M. (1999). Complete analysis of genes and enzymes for γ-hexachlorocyclohexane degradation in Sphingomonas paucimobilis UT26. Journal of Industrial Microbiology & Biotechnology, 23, 380–390.
Lal, R., Pandey, G., Sharma, P., Kumari, K., Malhotra, S., Pandey, R., et al. (2010). Biochemistry of microbial degradation of hexachlorocyclohexane and prospects for bioremediation. Microbiology and Molecular Biology Reviews, 74, 58–80.
Tabata, M., Endo, R., Ito, M., Ohtsubo, Y., Kumar, A., Tsuda, M., et al. (2011). The lin genes for γ-hexachlorocyclohexane degradation in Sphingomonas sp. MM-1 proved to be dispersed across multiple plasmids. Bioscience, Biotechnology, and Biochemistry, 75, 466–472.
Raina, V., Hauser, A., Buser, H. R., Rentsch, D., Sharma, P., Lal, R., et al. (2007). Hydroxylated metabolites of β- and δ-hexachlorocyclohexane: bacterial formation, stereochemical configuration, and occurrence in groundwater at a former production site. Environmental Science & Technology, 41, 4292–4298.
Wu, J., Hong, Q., Han, P., He, J., & Shunpeng, L. (2007). A gene linB2 responsible for the conversion of β-HCH and 2,3,4,5,6-pentachlorocyclohexanol in Sphingomonas sp. BHC-A. Applied Microbiology and Biotechnology, 73, 1097–1105.
Audus, L. J., & Symonds, K. V. (1955). Further studies on the breakdown of 2,4-D by a soil bacterium. Annals of Applied Biology, 42, 174–182.
Loos, M. A., Roberts, R. N., & Alexander, M. (1967). Phenols as intermediates in the decomposition of phenoxyacetates by an Arthrobacter species. Canadian Journal of Microbiology, 13, 679–690.
Tomati, U., Lippi, D., & Pietrosanti, W. (1970). Un complexe enzimatique capable de dégrader le 2,4-D. Mededelingen Faculteit Landbouw, Wetenschappen Gent, 35, 829–838.
Li, Q., Li, Y., Zhu, X. & Cai, B. (2008). Isolation and characterization of atrazine-degrading Arthrobacter sp. AD26 and use of this strain in bioremediation of contaminated soil. Journal of Environmental Sciences, 20, 1226–1230.
Wang, P., Qu, Y., & Zhou, J. (2009). Biodegradation of mixed phenolic compounds under high salt conditions and salinity fluctuations by Arthrobacter sp. W1. Applied Biochemistry and Biotechnology, 159, 623–633.
Sahoo, N. K., Pakshirajan, K., & Ghosh, P. K. (2011). Batch biodegradation of para-nitrophenol using Arthrobacter chlorophenolicus A6. Applied Biochemistry and Biotechnology, 165, 1587–1596.
Cacciari, I., Giovannozzi-Sermanni, G., Grappelli, A., & Lippi, D. (1971). Nitrogen fixation by Arthrobacter sp. I-Taxonomic study and evidence of nitrogenase activity of two new strains. Annali di Microbiologia ed Enzimologia, 21, 97–105.
Grappelli, A., & Rossi, W. (1979). Effect of herbicides on growth, respiration and IAA biosynthesis in Arthrobacter sp. Chemosphere, 6, 377–382.
Bianconi, D., De Paolis, M. R:, Agnello, A. C., Lippi, D., Pietrini, F., Zacchini, M., Polcaro, C., Donati, E., Paris, P., Spina, S. & Massacci, A. (2010). Field-scale rhizoremediation of a contaminated soil with hexachlorocyclohexane (HCH) isomers: the potential of poplars for environmental restoration and economical sustainability. In: Golubev, I. A. (ed.), Handbook of Phytoremediation, ch. 31. New York: Nova Science Publishers, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Paolis, M.R., Lippi, D., Guerriero, E. et al. Biodegradation of α-, β-, and γ-Hexachlorocyclohexane by Arthrobacter fluorescens and Arthrobacter giacomelloi . Appl Biochem Biotechnol 170, 514–524 (2013). https://doi.org/10.1007/s12010-013-0147-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0147-9