Abstract
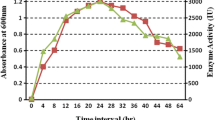
In the present study, we report the optimisation of batch conditions for improved α-1,4-glucan-glucanohydrolase (GGH) secretion by a nitrous acid (NA)-treated Bacillus alcalophilus. The wild (isolate GCB-18) and NA-derivative (mutant GCBNA-4) were grown in a medium containing 10 g/L nutrient broth, 10 g/L starch, 5 g/L lactose, 2 g/L ammonium sulphate, 2 g/L CaCl2 and phosphate buffer (pH 7.6). Sodium dodecyl sulphate (SDS) was used as an enzyme inducer while batch fermentations were carried out at 40 °C. The mutant produced GGH in 40 h which was 15-fold higher than the wild in presence of SDS. Thermodynamic studies revealed that the mutant culture exhibited the capability for improved enzyme activity over a broad range of temperature (35–70 °C). The enzyme was purified by cation-exchange column chromatography with ∼80 % recovery. The performance of fuzzy-logic system control was found to be highly promising for the improved substrate conversion rate. The correlation (1.045E + 0025) among variables demonstrated the model terms as highly significant indicating commercial utility of the culture used (P < 0.05).






Similar content being viewed by others
References
Ahmad, A., Jamshid, K. C., & Miland, L. (2010). International Journal of Macromolecular, 46, 289–297.
Brumm, P. J., & Teague, W. M. (1989). Biotechnology Letters, 11, 541–544.
Tester, R. F., Qi, X., & Karkalas, J. (2006). Animal Feed Science and Technology, 130, 39–54.
Baks, T., Bruins, M. E., Master, A. M., Janssen, A. E. M., & Boom, R. M. (2008). Journal of Agricultural and Food Chemistry, 56, 488–495.
Kolawole, A. O., Ajele, J. O., & Sirdeshmukh, R. (2011). Process Biochemistry, 46, 2178–2186.
Hyland, K., & Clayton, P. T. (1992). Clinical Chemistry, 38, 2405–2410.
Kasperski, A., & Miskiewicsz, T. (2002). Biotechnology Letters, 24, 17–21.
Malhotra, R., Noorwez, S., & Satyanaryana, T. (2000). Letters in Applied Microbiology, 31, 378–384.
Gun, E., Ster, S. C., Alexnder, A., & Gungar, N. (2004). Journal of Mathematical Sciences, 27, 17–22.
Rick, W., & Stegbauer, H. P. (1974). Methods of enzymatic analysis (2nd ed., Vol. 2, pp. 123–139). New York: Academic.
Bradford, M. M. (1976). Analytical Biochemistry, 72, 248–254.
Aiba, S., Humphrey, A. E., & Millis, N. F. (1973). Biochemical engineering (2nd ed., pp. 16–28). New York: Academic.
Nandi, S., Rahman, I., Kulkarni, S. G., Tambe, S. S., & Kulkarni, B. D. (2003). Chemical Bioprocess Engineering, 2, 773–779.
Snedecor, G. W., & Cochran, W. G. (1980). Statistical methods (7th ed., pp. 116–122). Iowa: Iowa State University Press.
Plackett, R. L., & Burman, J. P. (1946). Biometals, 33, 305–325.
Esfahani, Z., Rostami, K., & Mirdamadi, S. S. (2008). Pakistan Journal of Biological Sciences, 11, 253–259.
Jin, F., Xianzhen, L., Chunzi, Z., Qiao, S., Hua, W., Ziqiang, L., et al. (1992). Journal of Genetics and Applied Microbiology, 38, 293–302.
Skolpap, W., Scharer, J. M., Douglas, P. L., & Moo, Y. M. (2004). Bioengineering and Bioscience, 86, 706–717.
Lealem, F., & Gashe, B. A. (1994). Journal of Applied Bacteriology, 77, 348–352.
Ulger, C., & Cirakoglu, C. (2001). World Journal of Microbiology and Biotechnology, 17, 93–94.
Declerck, N., Machius, M., Joyet, P., Wiegand, G., Huber, R., & Gaillardin, C. (2003). Protein Engineering, 16, 287–293.
Li, X., & Yu, H. Y. (2011). Journal of Industrial Microbiology and Biotechnology, 38, 1837–1843.
Leman, P., Goesaert, H., & Delcour, J. A. (2009). Food Hydrocolloids, 23, 153–164.
Ahuja, S. K., Ferreira, G. M., & Morreira, A. R. (2004). Biotechnology and Bioengineering, 85, 666–675.
Acknowledgments
Chairman, Department of Botany is thanked for his assistance and moral support. All authors contributed equally in this work. The major part of the work was carried out and completed at Biotechnology Research Centre, Department of Botany. This research received no specific grant from any funding agency in the public, commercial or not for profit sectors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shamim, N., Ali, S. & Ikram-Ul-Haq Sodium Dodecyl Sulphate, a Strong Inducer of Thermostable Glucanhydrolase Secretion from a Derepressed Mutant Strain of Bacillus alcalophilus GCBNA-4. Appl Biochem Biotechnol 169, 2467–2477 (2013). https://doi.org/10.1007/s12010-013-0139-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0139-9