Abstract
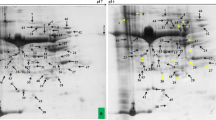
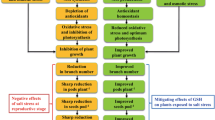
The present investigation was conducted to evaluate salt tolerance in ten genotypes of soybean (Glycine max L.). Twelve-day-old seedlings, grown hydroponically, were treated with 0, 25, 50, 75, 100, 125 and 150 mM NaCl for 10 days. Growth, lipid peroxidation and antioxidant enzyme activities were evaluated. Growth, measured in terms of length, fresh weight and dry weight of plants, was drastically reduced in Pusa-24 while there was little effect of NaCl treatment on Pusa-37 genotype of soybean. High level of lipid peroxidation was observed in Pusa-24 as indicated by increased level of malondialdehyde. Activities of superoxide dismutase, catalase, ascorbate peroxidase and glutathione reductase were maximum in Pusa-37 where 9-, 1-, 5- and 6-fold increase over control were observed, respectively. The results suggested that Pusa-24 and Pusa-37 are salt-sensitive and salt-tolerant genotype of soybean, respectively, and antioxidant defence system is involved in conferring the sensitiveness and tolerance in these genotypes. Salt-tolerant genotype Pusa-37, was further analysed by 2-dimensional gel electrophoresis to analyse the differential expression of proteins at high salt stress. In the present study, 173 protein spots were identified. Of these, 40 proteins were responsive to salinity in that they were either up- or downregulated. This study could help us in identifying the possible regulatory switches (gene/s) controlling novel proteins of the salt-tolerant genotype of the crop plants and their possible role in defence mechanism.









Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- BSA:
-
Bovine serum albumin
- CAT:
-
Catalase
- DW:
-
Dry weight
- EDTA:
-
Ethylenediaminetetraacetic
- FW:
-
Fresh weight
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidised glutathione
- MDA:
-
Malondialdehyde
- NBT:
-
Nitroblue tetrazolium
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
- MALDI-TOF-MS:
-
Matrix-assisted laser desorption ionisation time-of-flight mass spectrometry
References
FAO. (2005). Global network on integrated soil management for sustainable use of salt-affected soils. Rome: FAO land and plant nutrition management service.
Nemoto, Y., & Sasakuma, T. (2002). Differential stress responses of early salt-stress responding genes in common wheat. Phytochemistry, 61, 129–133.
Locy, R. D., Chang, C. C., Nielsen, B. L. & Singh, N. K. (1996). Photosynthesis in salt-adapted heterotrophic tobacco cells and regenerated plants. Plant Physiology, 110, 321–328.
Singh, R. K., Ghosh, P. K., Bandyopadhyay, K. K., Misra, A. K., Mandal, K.G. & Hati, K. M. (2006). Integrated plant nutrient supply for sustainable production in soybean – based cropping system. Indian Journal of Fertilizers 1(11), 25–32.
Hoagland, D. R., & Arnon, D. S. (1950). The water culture method for growing plants without soil. California Agricultural Experiment Station Circular, 347, 1–32.
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts I. Kinetic and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125, 189–198.
Bates, L. S., Walden, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39, 205–207.
Beauchamp, C. O., & Fridovich, I. (1971). Superoxide dismutase: improved assays and assay applicable to acrylamide gels. Analytical Biochemistry, 44, 276–287.
Giannopolitis, C. N., & Ries, S. K. (1977). Superoxide dismutase. I. Occurrence in higher plants. Plant Physiology, 59, 309–314.
Nakano, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22, 867–880.
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126.
Foyer, C. H., & Halliwell, B. (1976). The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta, 133, 21–25.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Damerval, C., Vienne, D. D., Zivy, M. & Thiellement, H. (1986). Technical improvements in two-dimensional electrophoresis increase the level of genetic variation detected in wheat-seedling proteins. Electrophoresis, 7, 52–54.
O’Farrell, P. H. (1975). High resolution two-dimensional electrophoresis of proteins. Journal of Biological Chemistry, 250, 4007–4021.
Chivasa, S., Ndimba, B. K., Simon, W. J., Robertson, D., Yu, X. L., Knox, J. P., Bolwell, P. & Slabas, A. R. (2002). Proteomic analysis of the Arabidopsis thaliana cell wall. Electrophoresis, 23, 1754–1765.
Laemilli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227 (5259), 680–685.
FAO (2000). Global network on integrated soil management for sustainable use of salt affected soils. Available from: http://www.fao.org/ag/AGL/agll/spush/intro.htm.
Munns, R. (2000). Comparative physiology of salt and water stress. Plant Cell and Environment, 25, 239–250.
Ruiz, J. M., Blasco, B., Rivero, R. M., & Romero, L. (2005). Nicotine-free and salt tolerant tobacco plants obtained by grafting to salinity-resistant root stocks of tomato. Physiologia Plantarum, 124, 465–475.
Greenway, H., & Munns, R. (1980). Mechanisms of salt tolerance in non-halophytes. Annual Reviews of Plant Physiology, 31, 149–190.
Gomez, J. M., Hernendez, J. A., Jimenez, A., del Rio, L. A., & Sevilla, F. (1999). Differential response of antioxidative enzymes of chloroplasts and mitochondria to long term NaCl stress of pea plants. Free Radical Research, 31, S11–S18.
Hernandez, J. A., Jimenez, A., Mullineaux, P., & Sevilla, F. (2000). Tolerance of pea (Pisum sativum L.) to a long term salt stress is associated with induction of antioxidant defences. Plant Cell and Environment, 23, 853–862.
Demiral, T., & Turkan, I. (2005). Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environmental and Experimental Botany, 53, 247–257.
Khan, M. H., Singh, L. B., & Panda, S. K. (2002). Changes in antioxidant levels in Oryza sativa L. roots subjected to NaCl-salinity stress. Acta Physiologia Plant, 24, 145–148.
Mandhania, S., Madan, S., & Sawhney, V. (2006). Antioxidant defense mechanism under salt stress in wheat seedlings. Biologia Plantarum, 227, 227–231.
Panda, S. K., & Khan, M. H. (2003). Salt stress influences lipid peroxidation and antioxidants in the leaf of an indica rice (Oryza sativa L). Physiology and Molecular Biology of Plants, 9, 273–278.
Panda, S. K., & Upadhyay, R. K. (2003). Salt stress injury induces oxidative alteration and antioxidative defense in the roots of Lemna minor. Biologia Plantarum, 48, 249–253.
Jain, M., Mathur, G., Koul, S., & Sarin, N. B. (2001). Ameliorative effects of proline on salt stress-induced lipid peroxidation in cell lines of groundnut (Arachis hypogaea L.). Plant Cell Reports, 20, 463–468.
Fadzilla, N. M., Finch, R. P., & Burdon, R. H. (1997). Salinity, oxidative stress and antioxidant responses in shoot cultures of rice. Journal of Experimental Botany, 48, 325–331.
Sairam, R. K., Srivastava, G. C., Agarwal, S., & Meena, R. C. (2005). Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biolgia Plantarum, 49, 85–91.
Willekens, H., Inze, D., Van Montagu, M., & Van Camp, W. (1995). Catalase in plants. Molecular Breeding, 1, 207–228.
Neto, A. D. A., Prisco, J. T., Eneás-Filho, J., Abreu, C. E. B., & Gomes-Filho, E. (2006). Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environmental and Experimental Botany, 56, 87–94.
Comba, M. E., Benavides, M. P., Gallego, S. M., & Tomaro, M. L. (1998). Relationship between nitrogen fixation and oxidative stress induction in nodules of salt treated soybean plants. Phyton-International Journal of Experimental Botany, 60, 115–126.
Bueno, P., Piqueras, A., Kurepa, J., Savoure, A., Verbruggen, N., Montagu, V. M., et al. (1998). Expression of antioxidant enzymes in response to abscisic acid and high osmoticum in tobacco BY-2 cell cultures. Plant Science, 138, 27–34.
Lechno, S., Zamski, E., & Tel-Or, E. (1997). Salt stress-induce responses in cucumber plants. Journal of Plant Physiology, 150, 206–211.
Sudhakar, C., Lakshmi, A., & Giridarakumar, S. (2001). Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Science, 161, 613–619.
Asada, K. (1999). The water–water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annual Reviews of Plant Physiology and Plant Molecular Biology, 50, 601–639.
Gueta-Dahan, Y., Yaniv, Z., Zilinskas, B. A., & Ben-Hayyim, G. (1997). Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in citrus. Planta, 203, 460–469.
Bor, M., Özdemir, F., & Türkan, I. (2003). The effect of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritima L. Plant Science, 164, 77–84.
Mittova, V., Volokita, M., Guy, M., & Tal, M. (2000). Activities of SOD and the ascorbate–glutathione cycle enzymes in subcellular compartments in leaves and roots of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennilli. Physiologia Plantarum, 110, 42–51.
Asada, K., & Takahashi, M. (1987). Photoinhibition. In D. J. Kyle, C. B. Osmond, & C. J. Arntzen (Eds.), Production and scavenging of active oxygen in photosynthesis (pp. 227–287). Amsterdam: Elsevier.
Delauney, A. J., & Verma, D. P. S. (1993). Proline biosynthesis and osmoregulation in plants. The Plant Journal, 4, 215–223.
Kavi-Kishor, P. B., Hong, Z., Miao, G. H., Hu, C. A. A., & Verma, D. P. S. (1995). Overexpression of D1-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiolgy, 108, 1387–1394.
Solomon, A., Beer, S., Waisel, Y., Jones, G. P., & Paleg, L. G. (1994). Effects of NaCl on the carboxylating activity of Rubisco from Tamarix jordanis in the presence and absence of proline-related compatible solutes. Physiologia Plantarum, 90, 198–204.
Van Rensburg, L., Kruger, G. H. J., & Kruger, H. (1993). Proline accumulation as drought-tolerance selection criterion: its relationship to membrane integrity and chloroplast ultrastructure in Nicotiana tabacum L. Journal of Plant Physiology, 141, 188–194.
Alia, Prasad, K. V. S. K., & Pardha-Saradhi, P. (1995). Effect of zinc on free radicals and proline in Brassica and Cajanus. Phytochemistry, 39, 45–47.
Venekamp, J. H. (1989). Regulation of cytosol acidity in plants under conditions of drought. Physiologia Plantarum, 70, 381–388.
Hagedorn, C. H., & Phang, J. M. (1986). Catalytic transfer of hydride ions from NADPH to oxygen by the inter conversions of proline and delta1-pyrroline-5-carboxylate. Archives of Biochemistry and Biophysics, 248, 166–174.
Ali, G., Srivastava, P. S., & Iqbal, M. (1999). Proline accumulation, protein pattern and photosynthesis in regenerants grown under NaCl stress. Biologia Plantarum, 42, 89–95.
Gzik, A. (1996). Accumulation of proline and pattern of amino acids in sugar beet plants in response to osmotic, water and salt stress. Environmental and Experimental Botany, 36, 29–38.
Petrusa, L., & Winicov, I. (1997). Proline status in salt tolerant and salt sensitive alfalfa cell lines and plants in response to NaCl. Plant Physiology and Biochemistry, 35, 303–310.
Bevan, M., Bancroft, I., Bent, E., Love, K., Goodman, H., Dean, C., et al. (1998). Analysis of 1.9Mb of contiguous sequence from chromosome 4 of Arabidopsis thaliana. Nature, 391, 485–488.
Witzel, K., Weidner, A., Surabhi, G. K., Börner, A. & Mock, H. P. (2009). Salt stress-induced alterations in the root proteome of barley genotypes with contrasting response towards salinity. Journal of Experimental Botany, 60, 3545–3557.
Abbasi, F. M., & Komatsu, S. (2004). A proteomic approach to analyze salt responsive proteins in rice leaf sheath. Proteomics, 4, 2072–2081.
Yan, S., Tang Z., Su, W. & Sun, W. (2005). Proteomic analysis of salt stress-responsive proteins in rice root. Proteomics, 5, 235–244
Shigeoka, S., Ishikawa, T., Tamoi, M., Miyagawa, Y., Takeda, T., Yabuta, Y., et al. (2002). Regulation and function of ascorbate peroxidase isoenzymes. Journal of Experimental Botany, 53, 1305–1319.
Jiang, Y., Yang, B., Harris, N. S. & Deyholos, M. K. (2007). Comparative proteomic analysis of NaCl stress-responsive proteins in Arabidopsis roots. Journal of Experimental Botany, 58, 3591–3607.
Caruso, G., Cavaliere, C., Guarino, C., Gubbiotti, R., Foglia, P. & Laganà, A. (2008). Identification of changes in Triticum durum L. leaf proteome in response to salt stress by two-dimensional electrophoresis and MALDI-TOF mass spectrometry. Analytical and Bioanalytical Chemistry, 391,381–390.
Wang, M. C., Peng, Z. Y., Li, C. L., Li, F., Liu, C. & Xia, G. M. (2008). Proteomic analysis on a high salt tolerance introgression strain of Triticum aestivum/Thinopyrum ponticum. Proteomics, 8, 1470–1489.
Author information
Authors and Affiliations
Corresponding author
Additional information
Khalid Rehman Hakeem, Faheema Khan and Ruby Chandna contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hakeem, K.R., Khan, F., Chandna, R. et al. Genotypic Variability Among Soybean Genotypes Under NaCl Stress and Proteome Analysis of Salt-Tolerant Genotype. Appl Biochem Biotechnol 168, 2309–2329 (2012). https://doi.org/10.1007/s12010-012-9939-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9939-6