Abstract
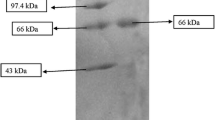
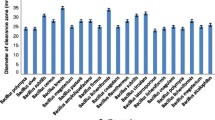
This study describes the characterization and optimization of medium components for an extracellular detergent, surfactant, organic solvent and thermostable serine alkaline protease produced by alkaliphilic Bacillus pumilus MCAS8 strain isolated from Pulicat lake sediments, Tamil Nadu, India. The strain yielded maximum protease (2,214 U/ml) under optimized conditions: carbon source, citric acid—1.5 % (w/w); inducer, soyabean meal—2 % (w/w); pH 11.0; shaking condition 37 °C for 48 h. The enzyme had pH and temperature optima of 9.0 and 60 °C, respectively. The enzyme displayed the molecular mass of 36 kDa in sodium dodecyl sulphate–polyacrylamide gel electrophoresis study and exhibited activity at a wide range of pH (6.0–11.0) and thermostability (20–70 °C). More than 70 % residual activity was observed when the enzyme was incubated with dithiothreitol, ethylenediaminetetraacetic acid, ethylene glycol tetraacetic acid and H2O2 for 30 min. The protease activity was also enhanced by divalent cations such as Ba2+, Ca2+ and Mg2+ and was strongly inhibited by Fe2+, Zn2+, Sr2+, Hg2+ and urea. The enzyme retained more than 50 % of its initial activity after pre-incubation for 1 h in the presence of 5 % (v/v) organic solvents such as dimethyl sulphoxide and acetone. The protease could hydrolyse various native proteinaceous substrates (1 % w/v) such as bovine serum albumin, casein, skim milk, gelatine, azocasein and haemoglobin. Wash performance analysis of enzyme revealed that it could effectively remove blood stains from the cotton fabric, thus making it suitable to use as an effective detergent additive. The protease enzyme also exhibited promising result in the dehairing of goat skin. The potency of the eco-friendly enzyme without using any chemicals against washing and dehairing showed that the enzyme could be used for various industrial applications.







Similar content being viewed by others
References
Kalisz, H. M. (1988). Microbial proteinases. Advances in Biochemical Engineering/Biotechnology, 36, 1–65.
Beg, Q. K., & Gupta, R. (2003). Purification and characterization of an oxidation-stable, thiol-dependent serine alkaline protease from Bacillus mojavensis. Enzyme and Microbial Technology, 32, 294–304.
Outtrup, H., & Boyce, C. O. L. (1990). Microbial proteinases and biotechnology. In C. T. Fogarty & K. Kelly (Eds.), Microbial enzymes and biotechnology (pp. 227–254). London: Elsevier.
Kumar, C. G., & Takagi, H. (1999). Microbial alkaline proteases: from a bioindustrial viewpoint. Biotechnology Advances, 17, 561–594.
Genckal, H., & Tari, C. (2006). Alkaline protease production from alkalophilic Bacillus sp. isolated from natural habitats. Enzyme Microbial Technology, 39, 703–710.
Jaouadi, B., Ellouz-Chaabouni, S., Ali, M. B., Messaoud, E. B., Naili, B., Dhouib, A., et al. (2009). Excellent laundry detergent compatibility and high dehairing ability of the Bacillus pumilus CBS alkaline proteinase (SAPB). Biotechnology and Bioprocess Engineering, 14, 503–512.
Haddar, A., Agrebi, R., Bougatef, A., Hmidet, N., Sellami-Kamoun, A., & Nasri, M. (2009). Two detergent stable alkaline serine-proteases from Bacillus mojavensis A21: purification, characterization and potential application as a laundry detergent additive. Bioresource Technology, 100, 3366–3373.
Maurer, K. H. (2004). Detergent proteases. Current Opinion in Biotechnology, 15, 330–334.
Li, S., He, B., Bai, Z., & Ouyang, P. (2009). A novel organic solvent-stable alkaline protease from organic solvent-tolerant Bacillus licheniformis YP1A. Journal of Molecular Catalysis B: Enzymatic, 56, 85–88.
Jaouadi, B., Ellouz-Chaabouni, S., Rhimi, M., & Bejar, S. (2008). Biochemical and molecular characterization of a detergent-stable serine alkaline protease from Bacillus pumilus CBS with high catalytic efficiency. Biochimie, 90, 1291–1305.
Wan, M. Y., Wang, H. Y., Zhang, Y. Z., & Feng, H. (2009). Substrate specificity and thermostability of the dehairing alkaline protease from Bacillus pumilus. Applied Biochemistry and Biotechnology, 159, 394–403.
Feng, Y., Yang, W., Ong, S., Hu, J., & Ng, W. (2001). Fermentation of starch for enhanced alkaline protease production by constructing an alkalophilic Bacillus pumilus strain. Applied Microbiology and Biotechnology, 57, 153–160.
Jaouadi, B., Aghajari, N., Haser, R., & Bejar, S. (2010). Enhancement of the thermostability and the catalytic efficiency of Bacillus pumilus CBS protease by site-directed mutagenesis. Biochimie, 92, 360–369.
Johnson, B. T., Shaw, L. N., Nelson, D. C., & Mayo, J. A. (2008). Extracellular proteolytic activities expressed by Bacillus pumilus isolated from endodontic and periodontal lesions. Journal of Medical Microbiology, 57, 643.
Kumar, C. G. (2002). Purification and characterization of a thermostable alkaline protease from alkalophilic Bacillus pumilus. Letters in Applied Microbiology, 34, 13–17.
Rahman, R. N. Z. R., Mahamad, S., Salleh, A. B., & Basri, M. (2007). A new organic solvent tolerant protease from Bacillus pumilus 115b. Journal of Industrial Microbiology and Biotechnology, 34, 509–517.
Wang, H. Y., Liu, D. M., Liu, Y., Cheng, C. F., Ma, Q. Y., Huang, Q., et al. (2007). Screening and mutagenesis of a novel Bacillus pumilus strain producing alkaline protease for dehairing. Letters in Applied Microbiology, 44, 1–6.
Hinman, R. L. (1994). The changing face of the fermentation industry. Chemtech, 24, 45–48.
Hadj-Ali, N. E., Agrebi, R., Ghorbel-Frikha, B., Sellami-Kamoun, A., Kanoun, S., & Nasri, M. (2007). Biochemical and molecular characterization of a detergent stable alkaline serine-protease from a newly isolated Bacillus licheniformis NH1. Enzyme and Microbial Technology, 40, 515–523.
McDonald, C. E., & Chen, L. L. (1965). The Lowry modification of the Folin reagent for determination of proteinase activity. Analytical Biochemistry, 10, 175.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Bergey, D. H., et al. (1984). Bergey’s manual of systematic bacteriology. Baltimore: Williams and Wilkins.
Christner, B. C., Mosley-Thompson, E., Thompson, L. G., & Reeve, J. N. (2001). Isolation of bacteria and 16S rDNAs from Lake Vostok accretion ice. Environmental Microbiology, 3, 570–577.
Altschul, S. F., Madden, T. L., SchSffer, A. A., Zhang, J., Zhang, Z., Miller, W., et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research, 25, 3389.
Najafi, M. F., Deobagkar, D., & Deobagkar, D. (2005). Potential application of protease isolated from Pseudomonas aeruginosa PD100. Electronic Journal of Biotechnology, 8, 79–85.
Giongo, J. L., Lucas, F. S., Casarin, F., Heeb, P., & Brandelli, A. (2007). Keratinolytic proteases of Bacillus species isolated from the Amazon basin showing remarkable de-hairing activity. World Journal of Microbiology and Biotechnology, 23, 375–382.
Johnvesly, B., & Naik, G. R. (2001). Studies on production of thermostable alkaline protease from thermophilic and alkaliphilic Bacillus sp. JB-99 in a chemically defined medium. Process Biochemistry, 37, 139–144.
Bajaj, B. K., & Sharma, P. (2011). An alkali-thermo-tolerant extracellular protease from a newly isolated Streptomyces sp. DP2. New Biotechnology. doi::10.1016/j.nbt.2011.01.001.
Divakar, K., Deepa, J., Priya, A., & Gautam, P. (2010). Purification and characterization of thermostable organic solvent-stable protease from Aeromonas veronii PG01. Journal of Molecular Catalysis B: Enzymatic, 66, 311–331.
Joo, H. S., Kumar, C. G., Park, G. C., Kim, K. T., Paik, S. R., & Chang, C. S. (2002). Optimization of the production of an extracellular alkaline protease from Bacillus horikoshii. Process Biochemistry, 38, 155–159.
Chu, W. H. (2007). Optimization of extracellular alkaline protease production from species of Bacillus. Journal of Industrial Microbiology and Biotechnology, 34, 241–245.
Laxman, R. S., et al. (2005). Optimization and scale up of production of alkaline protease from Conidioboluis coronatus. Process Biochemistry, 40, 3152–3158.
Boominadhan, U., Rajakumar, R., Sivakumaar, P. K. V., & Joe, M. M. (2009). Optimization of protease enzyme production using Bacillus sp. isolated from different wastes. Journal of Botanical Research International, 2, 83–87.
Dutta, J. R., Dutta, P. K., & Banerjee, R. (2005). Modeling and optimization of protease production by a newly isolated Pseudomonas sp. using a genetic algorithm. Process Biochemistry, 40, 879–884.
Liu, S., Fang, Y., Lv, M., Wang, S., & Chen, L. (2010). Optimization of the production of organic solvent-stable protease by Bacillus sphaericus DS11 with response surface methodology. Bioresource Technology, 101, 7924–7929.
Ping, W., Zhenming, C., & Chunling, M. A. (2006). Alkaline protease production by a strain of marine yeasts. Journal of Ocean University of China (English Edition), 5, 263–268.
Abidi, F., Liman, F., & Nejib, M. M. (2008). Production of alkaline proteases by Botrytis cinerea using economic raw materials: assay as biodetergent. Process Biochemistry, 43, 1202–1208.
Kanekar, P. P., Nilegaonkar, S. S., Sarnaik, S. S., & Kelkar, A. S. (2002). Optimization of protease activity of alkaliphilic bacteria isolated from an alkaline lake in India. Bioresource Technology, 85, 87–93.
Shanmughapriya, S., Krishnaveni, J., Selvin, J., Gandhimathi, R., Arunkumar, M., Thangavelu, T., et al. (2008). Optimization of extracellular thermotolerant alkaline protease produced by marine Roseobacter sp. (MMD040). Bioprocess and Biosystem Engineering, 31, 427–433.
Saeki, K., Ozaki, K., Kobayashi, T., & Ito, S. (2002). A novel species of alkaliphilic Bacillus that produces an oxidatively stable alkaline serine protease. Extremophiles, 6, 65–72.
Sivasubramanian, S., Manohar, B. M., Rajaram, A., & Puvanakrishnan, R. (2008). Ecofriendly lime and sulfide free enzymatic dehairing of skins and hides using a bacterial alkaline protease. Chemosphere, 70, 1015–1024.
Frikha, B., Kamoun, A., & Nasri, M. (2003). Stability studies of protease from Bacillus cereus BG1. Enzyme and Microbial Technology, 32, 513–518.
Joo, H. S., Kumar, C. G., Park, G. C., Paik, S. R., & Chang, C. S. (2004). Bleach-resistant alkaline protease produced by a Bacillus sp. isolated from the Korean polychaete, Periserrula leucophryna. Process Biochemistry, 39, 1441–1447.
Deng, A., Wu, J., Zhang, Y., Zhang, G., & Wen, T. (2010). Purification and characterization of a surfactant-stable high-alkaline protease from Bacillus sp. B001. Bioresource Technology, 101, 7100–7106.
Joo, H. S., & Chang, C. S. (2005). Production of protease from a new alkalophilic Bacillus sp. I-312 grown on soybean meal: optimization and some properties. Process Biochemistry, 40, 1263–1270.
Rao, M. B., Tanksale, A. M., Ghatge, M. S., & Deshpande, V. V. (1998). Molecular and biotechnological aspects of microbial proteases. Microbiology and Molecular Biology Reviews, 62, 597.
Li, W. F., Zhou, X. X., & Lu, P. (2005). Structural features of thermozymes. Biotechnology Advances, 23, 271–281.
Kazan, D., Denizci, A. A., Oner, M. N., & Erarslan, A. (2005). Purification and characterization of a serine alkaline protease from Bacillus clausii GMBAE 42. Journal of Industrial Microbiology and Biotechnology, 32, 335–344.
Rai, S. K., Roy, J. K., & Mukherjee, A. K. (2010). Characterisation of a detergent-stable alkaline protease from a novel thermophilic strain Paenibacillus tezpurensis sp. nov. AS-S24-II. Applied Microbiology and Biotechnology, 85, 1437–1450.
Chaphalkar, S. R., & Dey, S. (1998). Thermostable alkaline metalloprotease from newly isolated alkalophilic Streptomyces diastaticus strain SS1. Indian Journal of Biochemistry and Biophysics, 35, 34–40.
Oberoi, R., Beg, Q. K., Puri, S., Saxena, R. K., & Gupta, R. (2001). Characterization and wash performance analysis of an SDS-stable alkaline protease from a Bacillus sp. World Journal of Microbial Biotechnology, 17, 493–497.
Acknowledgments
The authors thank the Council for Scientific and Industrial Research, Government of India, New Delhi, India for financial assistance through a research project under Coastal Hazard Preparedness [No. 23 (0010)/07/EMR-11]. The authors also thank Ms. S. Devilakshmi, research scholar, Department of Biotechnology, IIT Madras, Chennai for her assistance in enzyme purification and zymogram analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayakumar, R., Jayashree, S., Annapurna, B. et al. Characterization of Thermostable Serine Alkaline Protease from an Alkaliphilic Strain Bacillus pumilus MCAS8 and Its Applications. Appl Biochem Biotechnol 168, 1849–1866 (2012). https://doi.org/10.1007/s12010-012-9902-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9902-6