Abstract
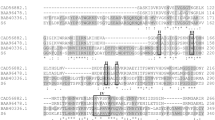
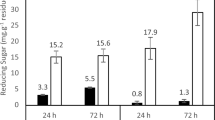
Alkaline pectate lyases are favorable for the textile industry. Here, we report the gene cloning and expression of a low-temperature-active alkaline pectate lyase (PL D) from Xanthomonas campestris ACCC 10048. Deduced PL D consists of a putative 27-residue signal peptide and a catalytic domain of 320 residues belonging to family PF09492. Recombinant PL D (r-PL D) produced in Escherichia coli was purified to electrophoretic homogeneity with a single step of Ni2+–NTA affinity chromatography and showed an apparent molecular weight of ~38 kDa. The pH and temperature optima of r-PL D were found to be 9.0 °C and 30 °C, respectively. Compared with its microbial counterparts, r-PL D had higher activity over a wide pH range (>45 % of the maximum activity at pH 3.0–12.0) and at lower temperatures (>35 % of activity even at 0 °C). The K m and V max values of r-PL D for polygalacturonic acid were 4.9 g l−1 and 30.1 μmol min−1 mg−1, respectively. Compared with the commercial compound pectinase from Novozymes, r-PL D showed similar efficacy in reducing the intrinsic viscosity of polygalacturonic acid (35.1 % vs. 36.5 %) and in bioscouring of jute (10.25 % vs. 10.82 %). Thus, r-PL D is a valuable additive candidate for the textile industry.






Similar content being viewed by others
References
Yadav, P. K., Singh, V. K., Yadav, S., Yadav, K. D., & Yadav, D. (2009). Biochemistry (Moscow), 74, 1049–1055.
Fogarty, W. M., & Ward, O. P. (1974). Progress in Industrial Microbiology, 13, 59–119.
Yuan, P., Meng, K., Luo, H., Shi, P., Huang, H., Bai, Y., et al. (2011). Process Biochemistry, 46, 1921–1926.
Beaulieu, C., Minsavage, G. V., Canteros, B. I., & Stall, R. E. (1991). Molecular Plant-Microbe Interactions, 4, 446–451.
Walker, S. G., & Ryan, M. E. (2003). FEMS Microbiology Letters, 226, 385–390.
Liao, C. H., Gaffney, T., Bradley, S., & Wong, L. (1996). Molecular Plant-Microbe Interactions, 9, 14–21.
Wing, R. A., Yamaguchi, J., Larabell, S. K., Ursin, V. M., & McCormick, S. (1990). Plant Molecular Biology, 14, 7–28.
Kovtunovych, G., Lar, O., Kamalova, S., Kordyum, V., Kleiner, D., & Kozyrovska, N. (1999). Plant and Soil, 215, 1–6.
Liao, C. (1991). Journal of Bacteriology, 173, 4386–4393.
Magro, P., Varvaro, L., Chilosi, G., Avanzo, C., & Balestra, G. (1994). FEMS Microbiology Letters, 117, 1–6.
Liao, C., Sasaki, K., Nagahashi, G., & Hicks, K. (1992). Molecular Plant-Microbe Interactions, 5, 301–308.
Heikinheimo, R., Flego, D., Pirhonen, M., Karlsson, M. B., Eriksson, A., Mäe, A., et al. (1995). Molecular Plant-Microbe Interactions, 8, 207–217.
Berensmeier, S., Singh, S., Meens, J., & Buchholz, K. (2004). Applied Microbiology and Biotechnology, 64, 560–567.
Chotigeat, W., Duangchu, S., Wititsuwannakun, R., & Phongdara, A. (2009). Plant Physiology and Biochemistry, 47, 243–247.
Hatada, Y., Kobayashi, T., & Ito, S. (2001). Extremophiles, 5, 127–133.
Hoondal, G., Tiwari, R., Tewari, R., Dahiya, N., & Beg, Q. (2002). Applied Microbiology and Biotechnology, 59, 409–418.
Collmer, A., Ried, J. L., & Mount, M. (1988). Methods in Enzymology, 161, 329–335.
Abdel-Halim, E. S., Konczewicz, W., Zimniewska, M., AI-Deyab, S. S., & EI-Newehy, M. H. (2010). Carbohydrate Polymers, 82, 195–201.
Novoa de Armas, H., Verboven, C., De Ranter, C., Desair, J., Vande Broek, A., Vanderleyden, J., et al. (2004). Acta Crystallographica Section D: Biological Crystallography, 60, 999–1007.
Vorhölter, F. J., Schneiker, S., Goesmann, A., Krause, L., Bekel, T., Kaiser, O., et al. (2008). Journal of Bacteriology, 134, 33–45.
Takao, M., Nakaniwa, T., Yoshikawa, K., Terashita, T., & Sakai, T. (2000). Bioscience, Biotechnology and Biochemistry, 64, 2360–2367.
Kobayashi, T., Hatada, Y., Higaki, N., Lusterio, D., Ozawa, T., Koike, K., et al. (1999). Biochimica et Biophysica Acta, 1427, 145–154.
Nasuno, S., & Starr, M. (1967). Biochemistry Journal, 104, 178–185.
Klug-Santner, B. G., Schnitzhofer, W., Vršanska, M., Weber, J., Agrawal, P., Nierstrasz, V., et al. (2006). Journal of Biotechnology, 121, 390–401.
Payasi, A., Sanwal, R., & Sanwal, G. G. (2008). World Journal of Microbiology and Biotechnology, 25, 1–14.
Margesin, R., Fauster, V., & Fonteyne, P. A. (2005). Letters in Applied Microbiology, 40, 453–459.
Ouattara, H. G., Reverchon, S., Niamke, S. L., & Nasser, W. (2011). Food Microbiology, 28, 2–8.
Xiao, Z., Boyd, J., Grosse, S., Beauchemin, M., Coupe, E., & Lau, P. C. (2008). Applied Microbiology and Biotechnology, 78, 973–981.
Saunders, N. F., Thomas, T., Curmi, P. M., Mattick, J. S., Kuczek, E., Slade, R., et al. (2003). Genome Research, 13, 1580–1588.
Sukhumsiirchart, W., Kawanishi, S., Deesukon, W., Chansiri, K., Kawasaki, H., & Sakamoto, T. (2009). Bioscience, Biotechnology and Biochemistry, 73, 268–273.
Leiros, H. K., Willassen, N. P., & Smalas, A. O. (1999). Extremophiles, 3, 205–219.
Thomas, T., & Cavicchioli, R. (1998). FEBS Letters, 439, 281–286.
Siddiqui, K. S., & Cavicchioli, R. (2006). Annual Review of Biochemistry, 75, 403–433.
Pissavin, C., Robert-Baudouy, J., & Hugouvieux-Cotte-Pattat, N. (1998). Biochimica et Biophysica Acta, 1383, 188–196.
Acknowledgments
This research was supported by the National Science and Technology Support Program (2011BADB02) and the National “948” Project (2011-G7-4) and the China Modern Agriculture Research System (CARS-42).
Author information
Authors and Affiliations
Corresponding author
Additional information
Peng Yuan and Kun Meng contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Yuan, P., Meng, K., Wang, Y. et al. A Low-Temperature-Active Alkaline Pectate Lyase from Xanthomonas campestris ACCC 10048 with High Activity over a Wide pH Range. Appl Biochem Biotechnol 168, 1489–1500 (2012). https://doi.org/10.1007/s12010-012-9872-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9872-8