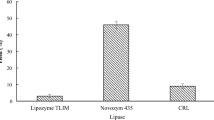
Abstract
Tannase has been extensively applied to synthesize gallic acid esters. Bioimprinting technique can evidently enhance transesterification-catalyzing performance of tannase. In order to promote the practical utilization of the modified tannase, a few enzymatic characteristics of the enzyme and its kinetic and thermodynamics properties in synthesis of propyl gallate by transesterification in anhydrous medium have been studied. The investigations of pH and temperature found that the imprinted tannase holds an optimum activity at pH 5.0 and 40 °C. On the other hand, the bioimprinting technique has a profound enhancing effect on the adapted tannase in substrate affinity and thermostability. The kinetic and thermodynamic analyses showed that the modified tannase has a longer half-time of 1,710 h at 40 °C; the kinetic constants, the activation energy of reversible thermal inactivation, and the activation energy of irreversible thermal inactivation, respectively, are 0.054 mM, 17.35 kJ mol−1, and 85.54 kJ mol−1 with tannic acid as a substrate at 40 °C; the free energy of Gibbs (ΔG) and enthalpy (ΔH) were found to be 97.1 and 82.9 kJ mol-1 separately under the same conditions.







Similar content being viewed by others
References
Chávez-González, M., Rodríguez-Durán, L. V., Balagurusamy, N., Prado-Barragán, A., Rodríguez, R., Contreras, J. C., & Aguilar, C. N. (2011). Biotechnological advances and challenges of tannase: An overview. Food Bioprocess Technology, 5, 445–459.
Aguilar, C. N., Rodriguez, R., Gutierrez-Sanchez, G., Augur, C., Favela-Torres, E., Prado-Barragan, L. A., Ramirez-Coronel, A., & Contreras-Esquivel, J. C. (2007). Microbial tannases: Advances and perspectives. Applied Microbiology and Biotechnology, 76, 47–59.
Jung, H. J., & Lim, C. J. (2011). The antiangiogenic and antinociceptive activities of n-propyl gallate. Phytotherapy Research, 25, 1570–1573.
Han, Y. H., Moon, H. J., You, B. R., Kim, S. Z., Kim, S. H., & Park, W. H. (2010). Propyl gallate inhibits the growth of HeLa cells via caspase-dependent apoptosis as well as a G1 phase arrest of the cell cycle. Oncology Reports, 23, 1153–1158.
Bracht, A., Eler, G. J., & Peralta, R. M. (2009). The action of n-propyl gallate on gluconeogenesis and oxygen uptake in the rat liver. Chemico-Biological Interactions, 181, 390–399.
Dolatabadi, J. E. N., & Kashanian, S. (2010). A review on DNA interaction with synthetic phenolic food additives. Food Research International, 43, 1223–1230.
Cheng, K. W., Yang, R. Y., Tsou, S. C. S., Lo, C. S. C., Ho, C. T., Lee, T. C., & Wang, M. F. (2009). Analysis of antioxidant activity and antioxidant constituents of Chinese toon. Journal of Functional Foods, 1, 253–259.
Fernandez-Lorente, G., Bolivar, J. M., Rocha-Martin, J., Curiel, J. A., Munoz, R., Rivas, B. D., Carrascosa, A. V., & Guisan, J. M. (2011). Synthesis of propyl gallate by transesterification of tannic acid in aqueous media catalysed by immobilised derivatives of tannase from Lactobacillus plantarum. Food Chemistry, 128, 214–217.
Raab, T., Bel-Rhlid, R., Williamson, G., Hansen, C. E., & Chaillot, D. (2007). Enzymatic galloylation of catechins in room temperature ionic liquids. Journal of Molecular Catalysis B: Enzymatic, 44, 60–65.
Gaathon, A., Gross, Z., & Rozhanski, M. (1989). Propyl gallate: enzymatic synthesis in a reverse micelle system. Enzyme and Microbial Technology, 11, 604–609.
Wang, G., Chen, Q., & Gu, W. (2010). Technical study on preparation of propyl gallate with tannin. Journal of Central South University of Forestry & Technology, 30, 137–140.
Yu, X. W., Li, Y. Q., Zhou, S. M., & Zheng, Y. Y. (2007). Synthesis of propyl gallate by mycelium-bound tannase from Aspergillus niger in organic solvent. World Journal of Microbiology and Biotechnology, 23, 1091–1098.
Weetall, H. H. (1985). Enzymatic synthesis of gallic acid esters. Applied Microbiology and Biotechnology, 11, 25–28.
Yu, X. W., Li, Y. Q., & Wu, D. (2004). Microencapsulation of tannase by chitosan−alginate complex coacervate membrane: Synthesis of antioxidant propyl gallate in biphasic media. Journal of Chemical Technology and Biotechnology, 79, 475–479.
Wang, Z. (2009). Synthesis of alky gallate by tannase immobilized in microemulsion-based-gels. Chemistry and Industry of Forest Products, 29, 1–5.
Sharma, S., & Gupta, M. N. (2003). Synthesis of antioxidant propyl gallate using tannase from Aspergillus niger van Teighem in nonaqueous media. Bioorganic & Medicinal Chemistry Letters, 13, 395–397.
Russel, A., & Klibanov, A. (1988). Inhibitor-induced enzyme activation in organic solvents. Journal of Biological Chemistry, 263, 11624–11626.
Fishman, A., & Cogan, U. (2003). Bio-imprinting of lipases with fatty acids. Journal of Molecular Catalysis B: Enzymatic, 22, 193–202.
Stahl, M., Jeppssonwistrand, U., Mansson, M. O., & Mosbach, K. (1991). Induced stereoselectivity and substrate selectivity of bio-imprinted Alpha-chymotrypsin in anhydrous organic media. Journal of the American Chemical Society, 113, 9366–9368.
Fernandez-Lorente, G., Palomo, J. M., Cabrera, Z., Fernandez-Lafuente, R., & Guisan, J. M. (2007). Improved catalytic properties of immobilized lipases by the presence of very low concentrations of detergents in the reaction medium. Biotechnology and Bioengineering, 97, 242–250.
Kamiya, N., Kasagi, H., Inoue, M., Kusunoki, K., & Goto, M. (1999). Enantioselective recognition mechanism of secondary alcohol by surfactant-coated lipases in nonaqueous media. Biotechnology and Bioengineering, 65, 227–232.
Thakar, A., & Madamwar, D. (2005). Enhanced ethyl butyrate production by surfactant coated lipase immobilized on silica. Process Biochemistry, 40, 3263–3266.
González-Navarro, H., & Braco, L. (1997). Improving lipase activity in solvent-free media by interfacial activation-based molecular bioimprinting. Journal of Molecular Catalysis B: Enzymatic, 3, 111–119.
Liu, T., Liu, Y., Wang, X. F., Li, Q., Wang, J. K., & Yan, Y. J. (2011). Improving catalytic performance of Burkholderia cepacia lipase immobilized on macroporous resin NKA. Journal of Molecular Catalysis B: Enzymatic, 71, 45–50.
Yang, J., Liu, L., & Cao, X. (2010). Combination of bioimprinting and silane precursor alkyls improved the activity of sol–gel-encapsulated lipase. Enzyme and Microbial Technology, 46, 257–261.
Teke, A. B., Sezginturk, M. K., Teke, M., Dinckaya, E., & Telefoncu, A. (2007). A bio-imprinted ascorbate oxidase biosensor. International Journal of Environmental and Analytical Chemistry, 87, 723–729.
Teke, M., Sezginturk, M. K., Dinckaya, E., & Telefoncu, A. (2008). A bio-imprinted urease biosensor: Improved thermal and operational stabilities. Talanta, 74, 661–665.
Kronenburg, N. A. E., de Bont, J. A. M., & Fischer, L. (2001). Improvement of enantioselectivity by immobilized imprinting of epoxide hydrolase from Rhodotorula glutinis. Journal of Molecular Catalysis B: Enzymatic, 16, 121–129.
Nie, G., Zheng, Z., Jin, W., Gong, G., & Wang, L. (2012). Development of a tannase biocatalyst based on bio-imprinting for the production of propyl gallate by transesterification in organic media. Journal of Molecular Catalysis B: Enzymatic, 78, 32–37.
Bhatti, H. N., & Saleem, N. (2009). Characterization of glucose oxidase from Penicillium notatum. Food Technology and Biotechnology, 47, 331–335.
Amin, M., Bhatti, H. A. Q. N., & Pervin, F. (2011). Production, partial purification and thermal characterization of β-amylase from Fusarium solani in solid state fermentation. Journal of the Chemical Society of Pakistan, 30, 480–485.
Shaheen, I., Bhatti, H. N., & Ashraf, T. (2008). Production, purification and thermal characterisation of invertase from a newly isolated Fusarium sp. under solid-state fermentation. International Journal of Food Science and Technology, 43, 1152–1158.
Ramos, E. L., Mata-Gomez, M. A., Rodriguez-Duran, L. V., Belmares, R. E., Rodriguez-Herrera, R., & Aguilar, C. N. (2011). Catalytic and thermodynamic properties of a tannase produced by Aspergillus niger GH1 grown on polyurethane foam. Applied Microbiology and Biotechnology, 165, 1141–1151.
Yu, X. W., & Li, Y. Q. (2006). Kinetics and thermodynamics of synthesis of propyl gallate by mycelium-bound tannase from Aspergillus niger in organic solvent. Journal of Molecular Catalysis B: Enzymatic, 40, 44–50.
Eyring, H., & Stearn, A. E. (1939). The application of the theory of absolute reaction rates to proteins. Chemical Reviews, 24, 253–270.
Cleland, W. W. (1963). The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochimica et Biophysica Acta, 67, 104–137.
King, E. L., & Altman, C. (1956). A schematic method of deriving the rate laws for enzyme-catalyzed reactions. Journal of Physical Chemistry, 60, 1375–1378.
Kasieczka-Burnecka, M., Kuc, K., Kalinowska, H., Knap, M., & Turkiewicz, M. (2007). Purification and characterization of two cold-adapted extracellular tannin acyl hydrolases from an Antarctic strain Verticillium sp. P9. Applied Microbiology and Biotechnology, 77, 77–89.
Battestin, V., & Macedo, G. A. (2007). Effects of temperature, pH and additives on the activity of tannase produced by Paecilomyces variotii. Electronic Journal of Biotechnology, 10, 191–199.
Mahapatra, K., Nanda, R. K., Bag, S. S., Banerjee, R., Pandey, A., & Szakacs, G. (2005). Purification, characterization and some studies on secondary structure of tannase from Aspergillus awamori nakazawa. Process Biochemistry, 40, 3251–3254.
Sabu, A., Kiran, G. S., & Pandey, A. (2005). Purification and characterization of tannin acyl hydrolase from Aspergillus niger ATCC 16620. Food Technology and Biotechnology, 43, 133–138.
Singh, R. S., Saini, G. K., & Kennedy, J. F. (2010). Covalent immobilization and thermodynamic characterization of pullulanase for the hydrolysis of pullulan in batch system. Carbohydrate Polymers, 81, 252–259.
Javed, M. R., Rashid, M. H., Nadeem, H., Riaz, M., & Perveen, R. (2009). Catalytic and thermodynamic characterization of endoglucanase (CMCase) from Aspergillus oryzae cmc-1. Applied Microbiology and Biotechnology, 157, 483–497.
Bhatti, H. N., Rashid, M. H., Nawaz, R., Khalid, A. M., Asgher, M., & Jabbar, A. (2007). Effect of aniline coupling on kinetic and thermodynamic properties of Fusarium solani glucoamylase. Applied Microbiology and Biotechnology, 73, 1290–1298.
Sharma, S., Bhat, T. K., & Dawra, R. K. (1999). Isolation, purification and properties of tannase from Aspergillus niger van Tieghem. World Journal of Microbiology and Biotechnology, 15, 673–677.
Acknowledgments
This work has been supported by grants Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-YW-G-050) and the National High Technology Research and Development Program of China (SQ2008AA02Z4477854).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nie, G., Zheng, Z., Gong, G. et al. Characterization of Bioimprinted Tannase and Its Kinetic and Thermodynamics Properties in Synthesis of Propyl Gallate by Transesterification in Anhydrous Medium. Appl Biochem Biotechnol 167, 2305–2317 (2012). https://doi.org/10.1007/s12010-012-9775-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9775-8