Abstract
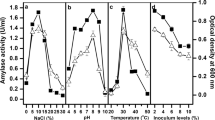
A haloalkaliphilic bacterium was isolated from salt-enriched soil of Mithapur, Gujarat (India) and identified as Bacillus agaradhaerens Mi-10-62 based on 16S rRNA sequence analysis (NCBI gene bank accession, GQ121032). The bacterium was studied for its α-amylase characteristic in the presence of organic solvents. The enzyme was quite active and it retained considerable activity in 30% (v/v) organic solvents, dodecane, decane, heptane, n-hexane, methanol, and propanol. At lower concentrations of solvents, the catalysis was quite comparable to control. Enzyme catalysis at wide range of alkanes and alcohol was an interesting finding of the study. Mi-10-62 amylase retained activity over a broader alkaline pH range, with the optimal pH at 10–11. Two molars of salt was optimum for catalysis in the presence of most of the tested solvents, though the enzyme retained significant activity even at 4 M salt. With dodecane, the optimum temperature shifted from 50 °C to 60 °C, while the enzyme was active up to 80 °C. Over all, the present study focused on the effect of organic solvents on an extracellular α-amylase from haloalkaliphilic bacteria under varying conditions of pH, temperature, and salt.




Similar content being viewed by others
References
Bonete, M. J., Camachmo, L., & Cadenaes. (1987). International Journal of Biochemistry, 19, 1149–1155.
Bonnete, F., Madern, D., & Zaccai, G. (1994). Journal of Molecular Biology, 244, 436–447.
Bernfeld, P. (1951). Interscience Publ., NY, 379.
Coronado, M. J., Carmen, V., Mellado, E., Tegos, G., Drainas, C., Nieto, J. J., et al. (2000). Microbiology, 146, 861–868.
Costa, D. A., Santos, M. S., & Galinski, E. A. (1998). Advances in Biochemical Engineering/Biotechnology, 61, 117–153.
Camacho, M. L., Brown, R. A., Bonete, M. J., Danson, M. J., & Hough, D. W. (1995). Isocitrate dehydrogenases from Haloferax volcanii and Sulfolobus solfataricus: enzyme purification, characterization and N-terminal sequence. FEMS Microbiology Letters, 134(1), 85–90.
Danson, M. J., & Hough, D. W. (1997). Biochemistry and Physiology, 117A, 307–312.
Dodia, M. S., Bhimani, H. G., Rawal, C. M., Joshi, R. H., & Singh, S. P. (2008). Bioresource Technology, 99, 6223–6227.
Dodia, M. S., Rawal, C. M., Bhimani, H. G., Joshi, R. H., Khare, S. K., & Singh, S. P. (2008). Journal of Industrial Microbiology and Biotechnology, 35(2), 121–132.
Dym, O., Mevarech, M., & Sussman, J. L. (1995). Science, 267, 1344–1346.
Eisenberg, H., Mevarech, M., & Zaccai, G. (1992). Advances in Protein Chemistry, 43, 1–62.
Eichler, J. (2001). Biotechnology Advances, 19, 261–278.
Good, W. A., & Paul, A. H. (1970). Journal of Bacteriology, 104(1), 601–603.
Gimenez, M. I., Studdert, C. A., Sanchez, J., & De Castro, R. (2000). Extremophiles, 4, 181–188.
Gupta, A., Roy, I., Patel, R. K., Singh, S. P., Khare, S. K., & Gupta, M. N. (2005). Journal of Chromatography. A, 1075, 103–108.
Herrera, S. K., Studdert, C., Sanchez, J., & De Castro, R. (1997). Journal of Basic Microbiology, 7, 313–322.
Herbert, R. A. (1992). Trends in Biotechnology, 10, 395–401.
Igrashi, K., Hatada, Y., Hagihara, H., Saeki, K., Takaiwa, M., Uemura, T., et al. (1998). Applied and Environmental Microbiology, 64, 3282–3289.
Jogi, C., Joshi, R. H., Dodia, M. S., & Singh, S. P. (2005). Journal of Cell and TissueResearch, 5(2), 439–444.
Joshi, R. H., Dodia, M. S., & Singh, S. P. (2008). Biotechnology and Bioprocess Engineering, 13, 552–559.
Karan, R., Singh, S. P., Kapoor, S. M., & Khare, S. K. (2010). New Biotechnology. doi:10.1016/j.nbt.2010.10.007.
Kadziola, A., Sogaard, M., Svensson, B., & Haser, R. (1998). Journal of Molecular Biology, 278, 205–217.
Lanyi, J. K. (1974). Bacteriological Reviews, 38, 272–290.
Nakamura, T., Syukunobe, Y., Sakarai, T., & Idota, T. (1993). Milchwissenschaft, 48, 11–14.
Marhuenda-Egea, F., & Bonete, M. J. (2002). Current Opinion in Biotechnology, 13, 385–389.
Madern, D., Ebel, C., & Zaccai, G. (2000). Extremophiles, 4, 91–98.
Mehta, V. J., Thumar, J. T., & Singh, S. P. (2006). Bioresource Technology, 97, 1650–1654.
Mijts, B. N., & Patel, B. K. C. (2001). Extremophiles, 5, 61–69.
Madigan, M. T., & Marrs, B. L. (1997). Scientific American, 276(4), 66–71.
Mevarech, M., Frolow, F., & Gloss, L. M. (2000). Biophysical Chemistry, 86, 155–164.
Martınez-Espinosa, R. M., Lledoo, B., Frutos Marhuenda-Egea, C., Dıaz, S., & Marıa Jose, B. (2009). Extremophiles, 13, 785–792.
Machius, M., Wiegand, G., & Huber, R. (1995). Journal of Molecular Biology, 246, 545–559.
Ogino, H., & Ishikawa, H. (2001). Journal of Bioscience and Bioengineering, 91, 109–116.
Patel, R. K., Dodia, M. S., & Singh, S. P. (2005). Process Biochemistry, 40, 3569–3575.
Patel, R. K., Dodia, M. S., Joshi, R. H., & Singh, S. P. (2006). World Journal of Microbiology and Biotechnology, 22(4), 375–382.
Ru, M. T., Dordick, J. S., Reimer, J. A., & Clark, D. S. (1999). Biotechnology and Bioengineering, 63(2), 233–241.
Studdert, C. A., Seitz, M. K. H., Gilv, M. I. P., Sanchez, J. J., & De Castro, R. (2001). Journal of Basic Microbiology, 41, 375–383.
Saraiva, J., Oliveira J., Hendrickx, M. (1996). Lebensmittel-Wissenschaft und-Technologie, 29, 310–315.
Tadamasa, F., Toru, M., Akinobu, E., Akira, I., & Usami, R. (2005). Extremophiles, 9, 85–89.
Thumar, J. T., & Singh, S. P. (2009). Journal of Industrial Microbiology and Biotechnology, 36, 211–218.
Thumar, J. T., & Singh, S. P. (2007). Journal of Chromatography B, 854, 198–203.
Vihinen, M., Peltonen, T., Iitia, A., Suominen, I., & Mantsala, P. (1994). Protein Engineering, 7, 1255–1259.
Upadek, H., & Kottwitz, B. (1997). Surfactant science series (pp. 203–212). New York: Marcel Dekker.
Wejse, P. L., Ingvorsen, K., & Mortensen, K. K. (2003). Extremophiles, 7, 423–431.
Acknowledgment
Financial assistance from the University Grants Commission (New Delhi, India) and Saurashtra University, Rajkot (India) is acknowledged. Mr. Sandeep Pandey is grateful to the UGC, New Delhi for the Research Fellowship in Sciences for Meritorious Students.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandey, S., Singh, S.P. Organic Solvent Tolerance of an α-Amylase from Haloalkaliphilic Bacteria as a Function of pH, Temperature, and Salt Concentrations. Appl Biochem Biotechnol 166, 1747–1757 (2012). https://doi.org/10.1007/s12010-012-9580-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9580-4