Abstract
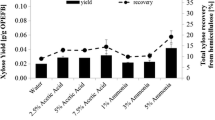
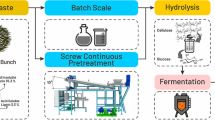
Empty fruit bunch (EFB), a residual product of the palm plantation, is an attractive biomass for biorefinery. As xylan is susceptible to high temperature pretreatment, it is important to setup a proper pretreatment condition to maximize the sugar recovery from EFB. Kinetic parameters of mathematical models were obtained in order to predict the concentration of xylose, glucose, furfural, and acetic acid in the hydrolysate and to find production conditions of xylose. We investigated the kinetics of hot liquid water and dilute sulfuric acid hydrolysis over a 40-min period using a self-designed setup by measuring the concentrations of released sugars (xylose, glucose) and degradation products (acetic acid and furfural). The reaction was performed within the range 160∼180 °C, under reaction conditions of various concentration of sulfuric acid (0.1∼0.2%) and 1:7 solid–liquid ratio in a batch reactor. The kinetic constants can be expressed by the Arrhenius equation with the activation energy for the hydrolysis of sugar and decomposition of sugar. The activation energy of xylose was determined to be 136.2187 kJ mol−1.




Similar content being viewed by others
Abbreviations
- A :
-
Acetic concentration (in grams per liter)
- A 0 :
-
Potential acetic concentration (in grams per liter)
- CXn 0 :
-
Initial composition of xylan
- E :
-
Activation energy (in kilojoules per mole)
- F :
-
Furfural concentration (in grams per liter)
- F 0 :
-
Potential furfural concentration (in grams per liter)
- HMF:
-
5-Hydroxymetyl-2-furfural
- k 1 :
-
Rate of generation reaction (per minute)
- k 2 :
-
Rate of decomposition reaction (per minute)
- k i :
-
Kinetic coefficient (per minute)
- k i0 :
-
Pre-exponential factor (per minute)
- LSR:
-
Liquid/solid ratio
- M :
-
Monomer concentration (in grams per liter)
- M 0 :
-
Initial monomer concentration (in grams per liter)
- P :
-
Polymer concentration (in grams per liter)
- P 0 :
-
Initial polymer concentration (in grams per liter)
- R :
-
Gas constant (8.314 × 10–3 kJ mol−1)
- T :
-
Temperature (in kelvin)
- t :
-
Time (in minutes)
References
Wyman, C. E. (1994). Bioresource Technology, 50, 3–16.
Chang, C., Cen, P., & Ma, X. (2007). Bioresource Technology, 98, 1448–1453.
Harris, J. F. (1975). Applied Polymer Symposium, 28, 131–140.
Lavarack, B. P., Griffin, G. J., & Rodman, D. (2002). Biomass Bioenergy, 23, 367–380.
Chen, W. H., Pen, B. L., Yu, C. T., & Hwang, W. S. (2011). Bioresource Technology, 102, 2916–2924.
Wahyudi, M., Sediawan, B., Sulistyo, H., & Hidayat, M. (2010). International Journal of Engineering Applied Science, 6, 64–69.
Rahman, S. H. A., Choudhury, J. P., & Ahmad, A. L. (2006). Biochemical Engineering Journal, 30, 97–103.
Garrote, G., Dominguez, H., & Parajo, J. C. (2001). Bioresource Technology, 79, 155–164.
Silva, S. S., Felipe, M. G. A., Silva, J. B. A., & Prata, A. M. R. (1998). Process Biochemistry, 33, 63–67.
Neureiter, M., Danner, H., Thomasser, C., Saidi, B., & Braun, R. (2002). Applied Biochemistry and Biotechnology, 98, 1–9.
Mohammed, M. A. A., Salmiaton, A., Wan Azlina, W. A. K. G., Mohammad Amran, M. S., Fakhru’l-Razi, A., & Taufiq-Yap, Y. H. (2011). Renewable and Sustainable Energy Reviews, 15, 1258–1270.
Malaysia Palm Oil Board (2010). www.mpob.gov.my. Accessed May 2010.
Omar, R., Idris, A., Yunus, R., Khalid, K., & Isma, M. I. A. (2011). Fuel, 90, 1536–1544.
Ruiz, R., Templeton, D., & Ehrman, T. (1996). Chemical analysis and testing standard procedure (NREL 1996; No.2-5). Golden: National Renewable Energy Laboratories.
Marquuardt, D. W. (1963). Journal of the Society for Industrial and Applied Mathematics, 11, 431–441.
Saeman, J. F. (1945). I&EC, 37, 43–52.
Lenihan, P., Orozco, A., O'Neill, E., Ahmad, M. N. M., Rooney, D. W., Mangwandi, C. and Walker, G. M. (2011). Biofuel production—recent developments and prospects. InTech. ISBN 978-953-307-478-8.
Gámez, S., Ramírez, J. A., Garrote, G., & Vázquez, M. (2004). Journal of Agricultural and Food Chemistry, 52, 4172–4177.
Rodriguez-Chonga, A., Ramirezb, J. A., Garrote, G., & Vázquez, M. (2004). Journal of Food Engineering, 61, 143–152.
Kim, S. B., & Lee, Y. Y. (1987). Biotechnology and Bioengineering Symposium, 17, 71–84.
Aguilar, R., Ramirez, J. A., Garrote, G., & Vazquez, M. (2002). Journal of Food Engineering, 55, 309–318.
Garrote, G., Dominguez, H., & Parajo, J. C. (1999). Journal of Chemical Technology and Biotechnology, 74, 1101–1109.
Garrote, G., Dominguez, H., & Parajo, J. C. (2001). Process Biochemistry, 36, 571–578.
Acknowledgments
This work was supported by the New & Renewable Energy of the Korea Institute of Energy Technology Evaluation and Planning grant funded by the Korea government Ministry of Knowledge Economy 2010T100100690.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J.S., Choi, W.I., Kang, M. et al. Kinetic Study of Empty Fruit Bunch Using Hot Liquid Water and Dilute Acid. Appl Biochem Biotechnol 167, 1527–1539 (2012). https://doi.org/10.1007/s12010-011-9528-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9528-0