Abstract
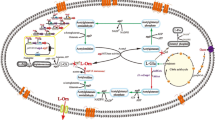
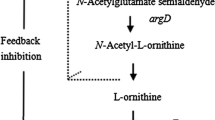
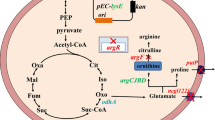
Ornithine acetyltransferase (EC 2.3.1.35; OATase) gene (argJ) from the l-arginine-producing mutant Corynebacterium crenatum SYPA5-5 was cloned, sequenced, and expressed in Escherichia coli BL21 (DE3). Analysis of the argJ sequence revealed that the argJ coded a polypeptide of 388 amino acids with a calculated molecular weight of 39.7 kDa. In this study, the function of the OATase (argJ) of C. crenatum SYPA5-5 has been identified as a conserved ATML sequence for the autolysis of the protein to α- and β-subunits. When the argJ regions corresponding to the α- and β-subunits were cloned and expressed separately in E. coli BL21, OATase activities were abolished. At the same time, a functional study revealed that OATase from C. crenatum SYPA5-5 was a bifunctional enzyme with the functions of acetylglutamate synthase (EC 2.3.1.1, NAGS) and acetylornithine deacetylase (EC 3.5.1.16, AOase) activities. In order to investigate the effects of the overexpression of the argJ gene on l-arginine production, the argJ gene was inserted into pJCtac to yield the recombinant shuttle plasmid pJCtac-argJ and then transformed into C. crenatum SYPA5-5. The results showed that the engineered strains could not only express more OATase (90.9%) but also increase the production of l-arginine significantly (16.8%).





Similar content being viewed by others
References
Lu, C. D. (2006). Applied Microbiology and Biotechnology, 70, 261–272.
Xu, H., Dou, W., Xu, H., Zhang, X., Rao, Z., Shi, Z., et al. (2009). Biochemical Engineering Journal, 43, 41–51.
Cunin, R., Glansdorff, N., Pierard, A., & Stalon, V. (1986). Microbiological Reviews, 50, 314–352.
Xu, Y., Labedan, B., & Glansdorff, N. (2007). Microbiology and Molecular Biology Reviews, 71, 36–47.
Rajagopal, B. S., DePonte, J., Tuchman, M., & Malamy, M. H. (1998). Applied and Environmental Microbiology, 64, 1805–1811.
Harris, B. Z., & Singer, M. (1998). Journal of Bacteriology, 180, 6412–6414.
Marc, F., Weigel, P., Legrain, C., Glansdorff, N., & Sakanyan, V. (2001). Journal of Biological Chemistry, 276, 25404–25410.
Crabeel, M., Abadjieva, A., Hilven, P., Desimpelaere, J., & Soetens, O. (1997). European Journal of Biochemistry, 250, 232–241.
Charlier, D. (2004). Biochemical Society Transactions, 32, 310–313.
Fernandez-Murga, M. L., & Rubio, V. (2008). Journal of Bacteriology, 90, 3018–3025.
Sakanyan, V., Petrosyan, P., Lecocq, M., Boyen, A., Legrain, C., Demarez, M., et al. (1996). Microbiology, 142(Pt 1), 99–108.
Ledwidge, R., & Blanchard, J. S. (1999). Biochemistry, 38, 3019–3024.
Shi, D., Morizono, H., Yu, X., Roth, L., Caldovic, L., Allewell, N. M., et al. (2005). Journal of Biological Chemistry, 280, 14366–14369.
Shinners, E. N., & Catlin, B. W. (1978). Journal of Bacteriology, 136, 131–135.
Martin, P. R., & Mulks, M. H. (1992). J Bacteriol, 174, 2694–2701.
Baetens, M., Legrain, C., Boyen, A., & Glansdorff, N. (1998). Microbiology, 144, 479–492.
Kimura, E. (2003). Advances in Biochemical Engineering/Biotechnology, 79, 37–57.
Hwang, G.-H., & Cho, J.-Y. (2010). Journal of Industrial Microbiology and Biotechnology, 37, 1131–1136.
Marc, F., Weigel, P., Legrain, C., Almeras, Y., Santrot, M., Glansdorff, N., et al. (2000). European Journal of Biochemistry, 267, 5217–5226.
Eikmanns, B. J., Thum-Schmitz, N., Eggeling, L., Ludtke, K. U., & Sahm, H. (1994). Microbiology, 140, 1817–1828.
Kirchner, O., & Tauch, A. (2003). Journal of Biotechnology, 104, 287–299.
Van der Rest, M. E., Lange, C., & Molenaar, D. (1999). Applied Microbiology and Biotechnology, 52, 541–545.
Xu, M. J., Rao, Z. M., Xu, H., Lan, C. Y., Dou, W. F., Zhang, X. M., et al. (2011). Applied Biochemistry and Biotechnology, 163, 707–719.
Ikeda, M., & Nakagawa, S. (2003). Appl Microbiol Biot, 62, 99–109.
Wendisch, V., Glansdorff, N., & Xu, Y. (2007). Microbial arginine biosynthesis: Pathway, regulation and industrial production (pp. 219–257). Berlin: Springer.
Abadjieva, A., Hilven, P., Pauwels, K., & Crabeel, M. (2000). Journal of Biological Chemistry, 275, 11361–11367.
Acknowledgments
This work was supported by Programs for New Century Excellent Talents in University (no. NCET-07-0380, NCET-10-0459), National Basic Research Program (973 Program) (no. 2007CB707804), and the National High-Tech Programs of China (no. 2006AA020104, 2006AA020301, 2007AA02Z207).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dou, W., Xu, M., Cai, D. et al. Improvement of l-Arginine Production by Overexpression of a Bifunctional Ornithine Acetyltransferase in Corynebacterium crenatum . Appl Biochem Biotechnol 165, 845–855 (2011). https://doi.org/10.1007/s12010-011-9302-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9302-3