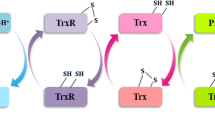
Abstract
Out of various tropical diseases caused by trypanosomatids, leishmaniasis is a life-threatening disease caused by the leishmania parasite. We are targeting the thiol metabolic pathway of the parasite for drug development, and trypanothione reductase (TryR) is a key enzyme of this pathway. It is important to gather significant knowledge about biophysical and intrinsic properties of this enzyme which will be helpful in better understanding of this drug-target enzyme. We report here the modulation of activity and stability of TryR from Leishmania infantum in the presence of various denaturants and pHs. The enzyme is quite stable under high concentration of denaturants and showed better stability compared to TryR of Leishmania donovani, whose sequence differs at only on position (Ala363→Gly). Structural basis of the destabilizing effects is discussed.





Similar content being viewed by others
References
Fairlamb, A. H., & Cerami, A. (1992). Annual Review of Microbiology, 46, 695–729.
Zhang, Y., Bond, C. S., Bailey, S., Cunningham, M. L., Fairlamb, A. H., & Hunter, W. N. (1996). Protein Science, 5, 52–61.
Desjeux, P. (1992). World Health Statistics, 45, 267–275.
Tempone, A. G., Borboremab, S. E., deAndrade, H. F., Amorim Gualda, N. C., Yogi, A., Carvalho, C. S., et al. (2005). Phytomedicine, 12, 382–390.
Delgado, J., Macias, J., Pineda, J. A., Corzo, J. E., Gonzalez-Moreno, M. P., de la Rosa, R., et al. (1999). American Journal of Medicine and Hygiene, 61, 766–769.
Henderson, G. B., Fairlamb, A. H., & Cerami, A. (1987). Molecular and Biochemical Parasitology, 24, 39–45.
Shukla, A. K., Singh, B. K., Patra, S., & Dubey, V. K. (2010). Applied Biochemistry and Biotechnology, 160, 2208–2218.
Ghisla, S., & Massey, V. (1989). European Journal of Biochemistry, 181, 1–17.
Borges, A., Cunningham, M. L., Tovar, J., & Fairlamb, A. H. (1995). European Journal of Biochemistry, 228, 745–752.
Fairlamb, A. H., Blackburn, P., Ulrich, P., Chait, B. T., & Cerami, A. (1985). Science, 227, 1485–1487.
Krauth-Siegel, R. L., Enders, B., Henderson, G. B., Fairlamb, A. H., & Schirmer, R. H. (1987). European Journal of Biochemistry, 164, 123–128.
Sarkar, N., Singh, A. N., & Dubey, V. K. (2009). Biological Chemistry, 390, 1057–1061.
Pompea, D. V., Giuseppe, G., Vincenzo, G., Guido, B., Luigi, M., Mose, R., et al. (2002). The Biochemical Journal, 367, 857–863.
Pace, C. N. (1975). CRC Crit. ReV. Biochem., 3, 1–43.
Rai, S., Dwivedi, U. N., & Goyal, N. (2009). Biochimica et Biophysica Acta, 1794, 1474–1484.
Baiocco, P., Colotti, G., Franceschini, S., & Ilari, A. (2009). Journal of Medicinal Chemistry, 52, 2603–2612.
Hamilton, C. J., Saravanamuthu, A., Eggleston, I. M., & Fairlamb, A. H. (2003). The Biochemical Journal, 369, 529–537.
Schonbrunn, E., Eschenburg, S., Luger, K., Kabsch, W., & Amrhein, N. (2000). Proceedings of the National Academy of Sciences of the United States of America, 97, 6345–6349.
Semisotnov, G. V., Rodionova, N. A., Razgulyaev, O. I., Uversky, V. N., Gripas, A. F., & Gilmanshin, R. I. (1991). Biopolymers, 31, 119–128.
Mayo, S. L., & Baldwin, R. L. (1993). Science, 262, 873–876.
Nandi, P. K., & Robinson, D. R. (1984). Biochemistry, 23, 6661–6668.
Acknowledgments
Infrastructural facilities and research fellowship to AKS by the Indian Institute of Technology Guwahati is acknowledged. Financial support by the Department of Biotechnology, Government of India in the form of research grant to VKD (Project No.: BT/PR10966/MED/29/88/2008) is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shukla, A.K., Patra, S. & Dubey, V.K. Biophysical and Folding Parameters of Trypanothione Reductase from Leishmania infantum . Appl Biochem Biotechnol 165, 13–23 (2011). https://doi.org/10.1007/s12010-011-9229-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9229-8