Abstract
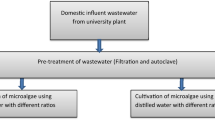
South Africa has a rich microalgal biodiversity which has the potential to be used for renewable bio-fuel production in the region. Bioprospecting for oleaginous microalgae in KwaZulu Natal Province, South Africa, resulted in the establishment of a microalgal culture collection system for alternative energy research in the country. A potential hyper-lipid-producing Chlorella spp. strain was isolated, purified, and cultured in supplemented post-chlorinated wastewater for biomass and lipid production at the laboratory scale under batch mode. The microalgal strain was cultivated in different strengths of BG-11 media supplemented with wastewater from a local municipal domestic wastewater treatment plant. The Chlorella spp. was grown using ambient dissolved carbon dioxide in shake flasks under photosynthetically active radiation (±120 μmolm−2s−1). Microalgal biomass and lipid productivity were monitored at 24-h intervals in the batch mode. The microalgal biomass was analyzed by direct light microscopy and indirectly by spectrophotometry at 600 nm, and the lipids were extracted and quantified. The growth rate of the Chlorella spp. was enhanced in post-chlorinated wastewater supplemented with 5 mM NaNO3 with maximal biomass productivity. A dramatic increase in lipid yield was achieved with the post-chlorinated wastewater supplemented with 25 mM NaNO3. Low dosages of free chlorine were found to enhance microalgal growth. These findings serve as a basis for further scale-up trials using municipal wastewater as a medium for microalgal biomass and lipid production.









Similar content being viewed by others
References
Basha, S. A., Gopal, K. R., & Jebaraj, S. (2009). A review on biodiesel production, combustion, emissions and performance. Renewable & Sustainable Energy Reviews, 13, 1628–1634.
Borowitzka, M. A. (2005). Culturing microalgae in outdoor ponds. In R. A. Andersen (Ed.), Algal culturing techniques (pp. 205–218). UK: Elsevier Academic.
Celekli, A., & Yavuzatmaca, M. (2009). Predictive modelling of biomass production by Spirulina platensis as function of nitrate and NaCl concentrations. Bioresource Technology, 100, 1847–1851.
Chinnasamy, S., Ramakrishnan, B., Bhatnagar, A., & Das, K. C. (2009). Biomass production potential of a wastewater alga Chlorella vulgaris ARC 1 under elevated levels of CO2 and temperature. International Journal of Molecular Sciences, 10, 518–532.
Chisti, Y. (2008). Biodiesel from microalgae beats bioethanol. Trends in Biotechnology, 26(3), 126–131.
Chisti, Y. (2007). Biodiesel from microalgae. Biotechnology Advances, 25, 294–306.
Costa, J. A. V., Colla, L. M., & Filho, P. D. (2003). Spirulina platensis growth in open raceway ponds using fresh water supplemented with carbon, nitrogen and metal ions. Zeitschrift für Naturforschung, 58, 76–80.
Danquah, M. K., Gladman, B., Moheimani, N., & Forde, G. M. (2009). Microalgal growth characteristics and subsequent influence on dewatering efficiency. Chemical Engineering Journal, 151, 73–78.
Eyster, C. (1958). Chloride effect on the growth of Chlorella pyrenoidosa. Nature, 181, 1141–1142.
Fajardo, A. R., Cerdán, L. S., Medina, A. R., Fernández, F. G. A., Moreno, P. A. G., & Grima, E. M. (2007). Lipid extraction from the microalga Phaeodactylum tricornutum. European Journal of Lipid Science and Technology, 109, 120–126.
Grima, E. M., Belarbi, E. H., Fernandez, F. G. A., Medina, A. R., & Chisti, Y. (2003). Recovery of microalgal biomass and metabolites: process options and economics. Biotechnology Advances, 20, 491–515.
Grobbelaar, J. U. (2007). Photosynthetic characteristics of Spirulina platensis grown in commercial-scale open outdoor raceway ponds: what do the organisms tell us? Journal of Applied Phycology, 19, 591–598.
Guillard, R. R. L. (2005). Purification methods for microalgae. In R. A. Andersen (Ed.), Algal culturing techniques (pp. 117–132). UK: Elsevier Academic.
Hsieh, C., & Wu, W. (2009). Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresource Technology, 100, 3921–3926.
Huang, G., Chen, G., & Chen, F. (2009). Rapid screening method for lipid production in alga based on Nile red fluorescence. Biomass and Bioenergy, 33(10), 1386–1392.
Huber, G. W., Iborra, S., & Corma, A. (2006). Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chemical Review, 106, 4044–4098.
Huesemann, M. H., Hausmann, T. S., Bartha, R., Aksoy, M., Weissman, J. C., & Benemann, J. R. (2009). Biomass productivities in wild type and pigment mutant of Cyclotella sp. (diatom). Applied Biochemistry and Biotechnology, 157, 507–526.
John, D. M. (2002). The freshwater algal flora of the British Isles: an identification guide to freshwater and terrestrial algae. London: Cambridge University Press.
Kong, Q., Li, L., Martinez, B., Chen, P., & Ruan, R. (2010). Culture of microalgae Chlamydomonas reinhardtii in wastewater for biomass feedstock production. Applied Biochemistry and Biotechnology, 160, 9–18.
Kott, Y., Hershkovitz, G., Shemtob, A., & Sless, J. B. (1966). Algicidal effect of bromine and chlorine on Chlorella pyredoidosa. Applied Microbiology, 14(1), 8–11.
Lee, S. J., Yoon, B., & Oh, H. (1998). Rapid method for the determination of lipid from the green alga Botryococcus braunii. Biotechnology Techniques, 12(7), 553–556.
Li, Q., Du, W., & Liu, D. (2008). Perspectives of microbial oils for biodiesel production. Applied Microbiology and Biotechnology, 80, 749–756.
Li, Y., Horsman, M., Wang, B., Wu, N., & Lan, C. Q. (2008). Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Applied Microbiology and Biotechnology, 81, 629–636.
Liu, Z., Wang, G., & Zhou, B. (2008). Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresource Technology, 99, 4717–4722.
Ma, F., & Hanna, M. A. (1999). Biodiesel production: a review. Bioresource Technology, 70, 1–15.
McGinnis, K. M., Dempster, T. A., & Sommerfeld, M. R. (1997). Characterization of the growth and lipid content of the diatom Chaetoceros mueller. Journal of Applied Phycology, 9, 19–24.
Moheimani, N. R., & Borowitzka, M. A. (2006). The long-term culture of the coccolithophore Pleurochrysis carterae (Haptophyta) in outdoor raceway ponds. Journal of Applied Phycology, 18, 703–712.
Mutanda, T., Ramesh, D., Karthikeyan, S., Kumari, S., Anandraj, A., & Bux, F. (2011). Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresource Technology, 102, 57–70. doi:10.1016/j.biortech.2010.06.077.
Olivier, S., Scragg, A. H., & Morrison, J. (2003). The effect of chlorophenols on the growth of Chlorella VT-1. Enzyme and Microbial Technology, 32, 837–842.
Pushparaj, B., Pelosi, E., Tredici, M. R., Pinzani, E., & Materassi, R. (1997). An integrated culture system for outdoor production of microalgae and cyanobacteria. Journal of Applied Phycology, 9, 113–119.
Rodolfi, L., Zittelli, G. C., Bassi, N., Padovani, G., Biondi, N., Bonini, G., et al. (2009). Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnology and Bioengineering, 102, 100–112.
Simmons, M. S., & Sivaborvorn, K. (1979). Effects of chlorinated organic from wastewater treatment on algal growth. Bulletin of Environmental Contamination and Toxicology, 23, 766–773.
Stanier, R. Y., Kunisawa, R., Mandel, M., & Cohen-Bazier, G. (1971). Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriological Reviews, 35, 171–205.
Stauber, J. L. (1997). Toxicity of chlorate to marine microalgae. Aquatic Toxicology, 41, 213–227.
Wang, L., Min, M., Li, Y., Chen, P., Chen, Y., Liu, Y., et al. (2010). Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Applied Biochemistry and Biotechnology. doi:10.1007/s12010-009-8866-7.
Widjaja, A., Chien, C., & Ju, Y. (2009). Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. Journal of the Taiwan Institute of Chemical Engineers, 40, 13–20.
Wijffels, R. H. (2007). Potential of sponges and microalgae for marine biotechnology. Trends in Biotechnology, 26(1), 26–31.
Acknowledgements
The authors would like to thank EThekwini Municipality, Economic Development Unit for funding this work and Mr. Cyprian Dlamini at the Kingsburgh Wastewater Treatment Plant, Durban, for free chlorine analysis of our water samples. The authors gratefully acknowledge the National Research Foundation for the KIC travel grant to New Delhi, India, for the 7th Asia-Pacific Conference on Algal Biotechnology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mutanda, T., Karthikeyan, S. & Bux, F. The Utilization of Post-chlorinated Municipal Domestic Wastewater for Biomass and Lipid Production by Chlorella spp. Under Batch Conditions. Appl Biochem Biotechnol 164, 1126–1138 (2011). https://doi.org/10.1007/s12010-011-9199-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9199-x