Abstract
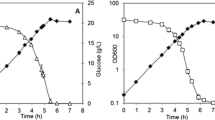
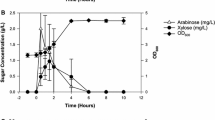
Agrobacterium sp. ATCC 31749 was previously shown to be an advantageous host for oligosaccharide production. Unexpectedly, the addition of citrate to the oligosaccharide synthesis reaction resulted in up to a sixfold improvement in the production N-aceytl-lactosamine, a disaccharide. The possible mechanisms for this citrate-induced stimulation of oligosaccharide production were investigated, including the consumption of citrate as a carbon and energy source, enhanced metal ion solubility from citrate chelation, and the ability of citrate to act as a buffer. The main mechanisms for the effect of citrate on oligosaccharide production were determined to be carbon and energy provision from citrate consumption and pH maintenance. ATCC 31749 was shown to co-metabolize citrate along with sucrose, a preferred carbon source, indicating the lack of a catabolite repression system in this Agrobacterium. Metabolic flux analysis suggested an increase in flux through TCA cycle for the citrate-containing reaction, which may provide additional energy supply to support enhanced oligosaccharide production. The citrate stimulation of oligosaccharide synthesis was shown to be unique to the Agrobacterium strain, as a similarly engineered Escherichia coli strain did not show significant improvement in oligosaccharide production with citrate addition. This work provides insight into the metabolism of Agrobacterium sp. ATCC 31749 and highlights important factors in whole-cell oligosaccharide synthesis.






Similar content being viewed by others
Abbreviations
- ACoA:
-
Acetyl coenzyme A
- ADP:
-
Adenosine diphosphate
- aKG:
-
Alpha-ketoglutarate
- ATP:
-
Adenosine triphosphate
- CIT:
-
Citrate
- Crd:
-
Curdlan
- ED:
-
Entner–Doudoroff
- F6P:
-
Fructose-6-phosphate
- FAD:
-
Flavin adenine dinucleotide (oxidized)
- FADH2 :
-
Flavin adenine dinucleotide (reduced)
- Fru:
-
Fructose
- G1P:
-
Glucose-1-phosphate
- G6P:
-
Glucose-6-phosphate
- GAP:
-
Glyceraldehyde-3-phosphate
- Glc:
-
Glucose
- Glycerol-3-P:
-
Glycerol-3-phosphate
- ICT:
-
Isocitrate
- LacNAc:
-
N-Aceytl-lactosamine
- MAL:
-
Malate
- NAD:
-
Nicotinamide adenine dinucleotide (oxidized)
- NADH:
-
Nicotinamide adenine dinucleotide (reduced)
- OAA:
-
Oxaloacetate
- P3:
-
Product 3 (galactose-β1,4-mannose)
- PPP:
-
Pentose phosphate pathway
- PTS:
-
Phosphotransferase system
- PYR:
-
Pyruvate
- SUCC:
-
Succinate
- SUCC-CoA:
-
Succinyl coenzyme A
- TCA:
-
Tricarboxylic acid
- UDP-Gal:
-
Uridine diphosphate galactose
- UDP-Glc:
-
Uridine diphosphate glucose
- UTP:
-
Uridine triphosphate
References
Dube, D. H., & Bertozzi, C. R. (2005). Nature Reviews. Drug Discovery, 4, 477–488.
Seeberger, P. H., & Werz, D. B. (2007). Nature, 446, 1046–1051.
Bettler, E., Imberty, A., Priem, B., Chazalet, V., Heyraud, A., Joziasse, D. H., et al. (2003). Biochemical and Biophysical Research Communications, 302, 620–624.
Bettler, E., Samain, E., Chazalet, V., Bosso, C., Heyraud, A., Joziasse, D. H., et al. (1999). Glycoconjugate Journal, 16, 205–212.
Mao, Z., Shin, H.-D., & Chen, R. R. (2006). Biotechnology Progress, 22, 369–374.
Padilla, L., Kramer, R., Stephanopoulos, G., & Agosin, E. (2004). Applied and Environmental Microbiology, 70, 370–376.
Priem, B., Gilbert, M., Wakarchuk, W. W., Heyraud, A., & Samain, E. (2002). Glycobiology, 12, 235–240.
Ruffing, A., Mao, Z., & Chen, R. R. (2006). Metabolic Engineering, 8, 465–473.
Kai, A., Arashida, T., Hatanaka, K., Akaike, T., Matsuzaki, K., Mimura, T., et al. (1994). Carbohydrate Polymers, 23, 235–239.
Phillips, K. R., & Lawford, H. G. (1983). in Progress in Industrial Microbiology, Vol. 18: Curdlan: Its properties and production in batch and continuous fermentations (D. E. Bushell), Elsevier, Amsterdam, pp 201–209.
Park, J. E., Lee, K. Y., Do, S. I., & Lee, S. S. (2002). Journal of Biochemistry and Molecular Biology, 35, 330–336.
Marier, J. R., & Boulet, M. (1958). Journal of Dairy Science, 41, 1683–1692.
Kim, M. K., Lee, I. Y., Lee, J. H., Kim, K. T., Rhee, Y. H., & Park, Y. H. (2000). Journal of Industrial Microbiology & Biotechnology, 25, 180–183.
Ko, Y.-T., & Lin, Y.-L. (2004). Journal of Agricultural and Food Chemistry, 52, 3313–3318.
Shedletzky, E., Unger, C., & Delmer, D. P. (1997). Analytical Biochemistry, 249, 88–93.
Arthur, L. O., Bulla, L. A., St. Julian, G., & Nakamura, L. K. (1973). Journal of Bacteriology, 116, 304–313.
Arthur, L. O., Nakamura, L. K., St. Julian, G., & Bulla, L. A. (1975). Applied Microbiology, 30, 731–737.
Fuhrer, T., Fischer, E., & Sauer, U. (2005). Journal of Bacteriology, 187, 1581–1590.
Park, J. E., Lee, K.-Y., Do, S.-I., & Lee, S. S. (2002). Journal of Biochemistry and Molecular Biology, 35, 330–336.
Koizumi, S., Endo, T., Tabata, K., Nagano, H., & Ozaki, A. (2000). Journal of Industrial Microbiology & Biotechnology, 25, 213–217.
Koizumi, S., Endo, T., Tabata, K., & Ozaki, A. (1998). Nature Biotechnology, 16, 847–850.
Gomori, G. (1955). Preparation of buffers for use in enzyme studies. New York: Academic.
Cozzone, A. J. (1998). Annual Review of Microbiology, 52, 127–164.
Goupry, S., Croguennec, T., Gentil, E., & Robins, R. J. (2000). FEMS Microbiology Letters, 182, 207–211.
Jyoti, B. D., Suresh, A. K., & Venkatesh, K. V. (2003). World Journal of Microbiology & Biotechnology, 19, 509–514.
Salou, P., Loubiere, P., & Pareilleux, A. (1994). Applied and Environmental Microbiology, 60, 1459–1466.
Sarantinopoulos, P., Kalantzopoulos, G., & Tsakalidou, E. (2001). Applied and Environmental Microbiology, 67, 5482–5487.
Schmitt, P., & Divies, C. (1991). Journal of Fermentation and Bioengineering, 71, 72–74.
Vaningelgem, F., Ghijsels, V., Tsakalidou, E., & De Vuyst, L. (2006). Applied and Environmental Microbiology, 72, 319–326.
Molin, G. (1985). Applied and Environmental Microbiology, 49, 1442–1447.
Ng, F. M.-W., & Dawes, E. A. (1973). The Biochemical Journal, 132, 129–140.
Acknowledgments
This work was funded by National Science Foundation (BES 0455193) and the American Cancer Society. A. Ruffing acknowledges a NSF graduate fellowship.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
-
(1)
Sucrose → Glc + Fru
-
(2)
Glc → Glc (extracellular)
-
(3)
Fru → Fru (extracellular)
-
(4)
Glc + ATP → G6P + ADP
-
(5)
Fru + ATP → F6P + ADP
-
(6)
F6P → G6P
-
(7)
G6P → G1P
-
(8)
G1P + ATP → UDP-Glc + ADP
-
(9)
UDP-Glc → Crd
-
(10)
UDP-Glc → UDP-Gal
-
(11)
UDP-Gal + Glc → Lactose
-
(12)
UDP-Gal + GlcNAc → LacNAc
-
(13)
UDP-Gal + F6P → P3
-
(14)
G6P → 6PG
-
(15)
6PG → GAP + PYR
-
(16)
Glycerol-3-P + NAD → GAP + NADH
-
(17)
Glycerol + ATP → Glycerol-3-P + ADP
-
(18)
GAP + 2 ADP + NAD → PYR + 2 ATP + NADH
-
(19)
PYR + NAD → ACoA + NADH
-
(20)
ACoA + ADP → Acetate + ATP
-
(21)
ACoA + OAA → CIT
-
(22)
CIT (extracellular) → CIT
-
(23)
CIT → ICT
-
(24)
ICT + NAD → aKG + NADH
-
(25)
aKG + NAD → SUCC-CoA + NADH
-
(26)
SUCC-CoA + ADP → SUCC + ATP
-
(27)
SUCC + FAD → MAL + FADH2
-
(28)
MAL + NAD → OAA + NADH
-
(29)
ICT + ACoA → SUCC + MAL
-
(30)
NADH + 3 ADP → NAD + 3 ATP
-
(31)
FADH2 + 2 ADP → FAD + 2 ATP
-
(32)
ATP → ADP
Rights and permissions
About this article
Cite this article
Ruffing, A.M., Chen, R.R. Citrate Stimulates Oligosaccharide Synthesis in Metabolically Engineered Agrobacterium sp.. Appl Biochem Biotechnol 164, 851–866 (2011). https://doi.org/10.1007/s12010-011-9179-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9179-1