Abstract
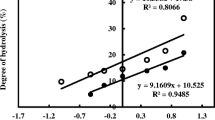
Four different commercial proteases (Protease-P-Amano6, Alcalase®, Protex 7L®, and Neutrase®) were evaluated for recovering lipids and protein simultaneously by hydrolysis. Fungal protease (Protease-P-Amano6) resulted in maximum lipid recovery (74.9%) followed by alcalase (61.7%). Peroxide value (PV; milli-equivalents of oxygen per kilogram) in the oil recovered after hydrolysis was 40.48 compared to 8.7 in lipids from fresh fish viscera. However, addition of tertiary butyl hydroxyl quinine at 200 ppm level maintained the PV of oil recovered by hydrolysis closer to oil from fresh waste. Degree of hydrolysis was the highest in case of fungal protease (49.1%) where neutrase resulted in higher total antioxidant activity (micrograms of ascorbic acid equivalents per milligram protein) of 34.4. Protein hydrolysate prepared using fungal protease had the higher diphenylpicrylhydrazyl radical scavenging activity as compared to those from other enzymes. The results indicate the utility of commercial proteases in providing an ecofriendly and feasible solution for reducing disposal problems associated with fish processing.




Similar content being viewed by others
References
FAO (2010). Year book of fishery statistics—latest summary of tables. Available at http://www.fao.org/fishery/statistics/en; last accessed on May 18, 2010.
Bhaskar, N., Sachindra, N. M., Suresh, P. V., Mahendrakar, N. S. (2010). Microbial reclamation of fish industry bi-products. In: D. Montet & RC. Ray (Eds.), Aquaculture microbiology (pp. 248–275). Science, Enfield.
Sachindra, N. M., Bhaskar, N., Hosokawa, M., & Miyashita, K. (2010). Value addition to fish processing by-products. In C. Alasalvar, K. Miyashita, U. Wanasundara, & F. Shahidi (Eds.), Seafood quality, safety and health applications (pp. 390–401). UK: Blackwell.
Vidotti, R. M., Viegas, E. M. M., & Careiro, D. J. (2003). Amino acid composition of processed fish silage using different raw materials. Animal Feed Science and Technology, 105, 199–204.
Swapna, H. C., Amit, K. R., Bhaskar, N., & Sachindra, N. M. (2010). Lipid classes and fatty acid profile of selected Indian fresh water fishes. Journal of Food Science and Technology, 47, 394–400.
Kim, S. K., & Mendis, E. (2006). Bioactive compounds from marine processing by-products—a review. Food Research International, 39, 383–393.
Song, L., Li, T., Yu, R., Yan, C., Ren, S., & Zhao, Y. (2008). Antioxidant activities of hydrolysates of Arca subcrenata prepared with three proteases. Marine Drugs, 6, 607–619.
Sikoroski, Z. E., & Naczk, M. (1981). Modification of technological properties of fish protein concentrate. CRC Critical Review Food Science and Nutrition, 14, 201–230.
Hoyle, N. T., & Merritt, J. H. (1994). Quality of fish protein hydrolysate from herring (Clupea harengus). Journal of Food Science, 59, 76–79.
Kristinssons, H. G., & Rasco, B. A. (2000). Fish protein hydrolysates: Production, biochemical and functional properties. Critical Reviews in Food Science and Nutrition, 40, 43–81.
Bhaskar, N., Benila, T., Radha, C., & Lalitha, R. G. (2008). Optimization of enzymatic hydrolysis of visceral waste proteins of catla (Catla catla) for preparing protein hydrolysate using a commercial protease. Bioresource Technology, 99, 335–343.
Bhaskar, N., & Mahendrakar, N. S. (2008). Protein hydrolysate from visceral waste proteins of catla (Catla catla): Optimization of hydrolysis conditions for a commercial neutral protease. Bioresource Technology, 99, 4105–4111.
Slizyte, R., Dauksas, E., Falch, E., Storro, I., & Rustad, T. (2005). Characteristics of protein fractions generated from hydrolysed cod (Gadus morhua) by-products. Process Biochemistry, 40, 2021–2033.
Grodji, A. G., Michel, L., Jacques, F., & Michel, P. (2006). Analysis of lipids extracted from salmon (Salmo salar) heads by commercial proteolytic enzymes. European Journal of Lipid Science and Technology, 108, 766–775.
AOAC. (2000). Official methods of analysis (17th ed.). Washington, DC: Association of Official Analytical Chemists.
Guerard, F., Dufosse, L., De, L., Broise, D., & Binet, A. (2001). Enzymatic hydrolysis of proteins from yellowfish tuna (Thunnus albacares) wastes using Alcalase. Journal of Molecular Catalysis. B, Enzymatic, 11, 1051–1059.
Amit, K. R., Swapna, H. C., Bhaskar, N., Halami, P. M., & Sachindra, N. M. (2010). Effect of fermentation ensilaging on recovery of oil from fresh water fish viscera. Enzyme and Microbial Technology, 46, 9–13.
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917.
Amit, K. R., Bhaskar, N., Halami, P. M., Indirani, K., Suresh, P. V., & Mahendrakar, N. S. (2009). Characterisation and application of a native lactic acid bacterium isolated from tannery fleshings for the fermentative bioconversion of tannery fleshing. Applied Microbiology and Biotechnology, 83, 757–766.
Statsoft. (1999). Statistica for Windows. Tulsa: Statsoft.
Sathivel, S., Prinyawiwatkul, W., Grimm, C. C., King, J. M., & Lloyd, S. (2002). FA composition of crude oil recovered from catfish viscera. Journal of the American Oil Chemist’s Society, 78, 989–992.
Klomklao, S., Benjakul, S., & Visessanguan, W. (2004). Comparative studies on proteolytic activity of splenic extract from three tuna species commonly used in Thailand. Journal of Food Biochemistry, 28, 355–372.
Ahmed, J., & Mahendrakar, N. S. (1996). Autolysis and rancidity development in fish viscera during fermentation. Bioresource Technology, 58, 247–251.
Shon, M. Y., Kim, T. H., & Sung, N. J. (2003). Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food Chemistry, 82, 593–597.
Sachindra, N. M., & Bhaskar, N. (2008). In vitro antioxidant activity of liquor from fermented shrimp biowaste. Bioresource Technology, 99, 9013–9016.
Acknowledgments
Authors thank Department of Biotechnology, Govt. of India for partial funding of this work through Grant ##BT/PR 9474/AAQ/03/345/2007. Authors place on record their thanks to Dr. V Prakash, Director, CFTRI for encouragement and permission to publish the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hathwar, S.C., Bijinu, B., Rai, A.K. et al. Simultaneous Recovery of Lipids and Proteins by Enzymatic Hydrolysis of Fish Industry Waste Using Different Commercial Proteases. Appl Biochem Biotechnol 164, 115–124 (2011). https://doi.org/10.1007/s12010-010-9119-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-010-9119-5