Abstract
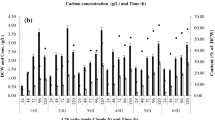
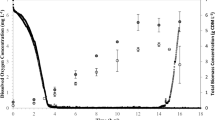
Polyhydroxyalkanoates (PHAs) are polymers of hydroxyalkanoate, which are accumulated by many bacteria as food storage material under excess carbon source and limited nitrogen source. In our study, Enterobacter cloacae SU-1 isolated from the rhizospheric soil of Arachis hypogea was allowed to grow as batch culture in minimal media containing either glucose or lactose, and the pattern of PHA accumulation by E. cloacae SU-1 was studied. E. cloacae SU-1 was found to accumulate 94% of PHA/dry weight of the organism in 8 g/l lactose-containing medium. When the monomeric units of PHA of E. cloacae SU-1 was analyzed by gas chromatography, it was also found to accumulate medium chain length PHA 3-hydroxyoctanoate (3HO)/3-hydroxyhexanoate (3HH) in the presence of glucose and lactose, but the ratio of these monomers differed as 11:1 and 6:1, respectively.






Similar content being viewed by others
References
Elbanna, K., Tina, L., Eversloh, K., Jendrossek, D., Luftmann, H., & Steinbuchel, A. (2004). Studies on the biodegradability of polythioester copolymers and homopolymers by polyhydroxyalkanoate (PHA)-degrading bacteria and PHA depolymerases. Archives of Microbiology, 182, 212–225.
Poirier, Y. (2002). Polyhydroxyalkanoate synthesis in plants as tool for biotechnology and basic studies of lipid metabolism. Progress in Lipid Research, 41, 131–155.
Page, W. J., Manchak, J., & Rudy, B. (1992). Formation of poly(hydroxybutyrae-co-valerate) by Azotobacter venelandii UWD. Applied and Environmental Microbiology, 58, 2866–2873.
Rehm, B. H. A., & Steinbuchel, A. (1999). Biochemical and genetic analysis of the PHA synthases and other proteins required for PHA synthesis. International Journal of Biological Macromolecules, 13, 83–88.
Langenbach, S., Rehm, B. H. A., & Steinbuchel, A. (1997). Functional expression of the PHA synthase gene phaC1 from Pseudomonas aeruginosa in Escherichia coli. Microbiol Biotechnology, 31, 329–333.
Qi, Q. S., Steinbüchel, A., & Rehm, B. H. A. (1998). Metabolic routing towards polyhydroxyalkanoic acid synthesis in recombinant Escherichia coli (fadR): Inhibition of fatty acid beta-oxidation by acrylic acid. FEMS Microbiology Letters, 167, 89–94.
Rehm, B. H. A., Kruger, N., & Steinbuchel, A. (1998). A new metabolic link between fatty acid de novo synthesis and polyhydroxyalkanoic acid synthesis. The Journal of Biological Chemistry, 273, 24044–24051.
Chan, P. L., Yu, V., Wai, L., & Yu, H. F. (2006). Production of medium-chain-length polyhydroxyalkanoatess by Pseudomonas aeruginosa with fatty acids and alternative carbon sources. Applied Biochemistry and Biotechnology, 6(129–132), 933–941.
Lakshman, K., & Shamala, T. R. (2003). Enhanced biosynthesis of polyhydroxyalkanoatess in a mutant strain of Rhizobium meliloti. Biotechnology Letters, 25, 115–119.
Potter, M., & Steinbuchel, A. (2005). Poly (3-hydroxybutrate) granule associated proteins: Impacts on poly(3-hydroxybutrate) synthesis and degradation. Biomacromolecules, 6, 552–560.
Pitcher, D. G., Saunders, N. A., & Owen, R. J. (1989). Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Letter Applied Microbiology., 8, 151–156.
Weisburg, W. G., Barns, S. M., Pelletier, D. A., & Lane, D. J. (1991). 16S ribosomal DNA amplification for phylogenetic study. Journal of Bacteriology, 173, 697–703.
Law, K. H., Leung, Y. C., Lawford, H., Chua, H., Lo, W. H., & Yu, P. H. (2001). Production of polyhydroxybutrate by Bacillus species isolated from municipal activated sludge. Applied Biochemistry and Biotechnology, 91–93, 515–524.
Omar, S., Rayes, A., Eqaab, A., VoB, I., & Steinbüchel, A. (2001). Optimization of cell growth and poly(3-hydroxybutyrate) accumulation on date syrup by a Bacillus megaterium strain. Biotechnology Letters, 23(14), 1119–1123.
Joshi, P. A., & Jaysawal, S. R. (2010). Isolation and characterization of poly-hydroxyalkanoate producing bacteria from sewage sample. Journal of Cell and Tissue Research, 10(1), 2165–2168.
Lageveen, R. G., Huisman, G. W., Preusting, H., Ketelaar, P., Eggink, G., & Witholt, B. (1988). Formation of polyesters by Pseudomonas oleovorans: effect of substrates on formation and composition of poly(R)-3-hydroxyalkanoates and poly(R)-3-hydroxyalkanoates. Applied and Environmental Microbiology, 54, 2924–2932.
Haywood, G. W., Anderson, A. J., Ewing, D. F., & Dawes, E. A. (1990). Accumulation of a polyhydroxyalkanoate containing primarily3-hydroxydecanoate from simple carbohydrate substrates by Pseudomonas sp. strain NCIMB40135. Applied and Environmental Microbiology, 56, 3354–3359.
Rehm, B. H. A. (2006). Genetics and biochemistry of polyhydroxyalkanoate granule self-assembly: The key role of polyester synthases. Biotechnology Letters, 28, 207–213.
Fukui, T., Shiomi, N., & Doi, Y. (1998). Expression and characterization R-specific enoyl coenzyme A hydratase involved in polyhydroxyalkanoate biosynthesis by Aeromonas caviae. Journal of Bacteriology, 180, 667–673.
Hoffmann, N., Amara, A. A., Beermann, B. B., Qi, Q., Hinz, H. J., & Rehm, B. H. A. (2002). Biochemical characterization of Pseudomonas putida 3-hydroxyacyl ACP: CoA transacylase, which diverts intermediates of fatty acids de novo biosynthesis. The Journal of Biological Chemistry, 277, 42926–42936.
Anderson, A. J., & Dawes, E. A. (1990). Occurrence, metabolism, metabolic role and industrial uses of bacterial polyhydroxyalkanoates. Microbiological Reviews, 54, 450–472.
Steinbuchel, A. (1991). Polyhydroxyalkanoaic acids. In D. Byrom (Ed.), Biomaterials: Novel materials from biological sources (pp. 124–213). New York: Stockton.
Steinbuchel, A., & Pieper, U. (1992). Production of a copolyester of 3-hydroxybutyric acid and 3-hydroxyvaleric acid from single unrelated carbon sources by a mutant of Alcaligenes eutrophus. Applied Microbiology and Biotechnology, 37, 1–6.
He, W. N., Tian, W. D., Zhang, G., & Chen, G. Q. (1998). Production of novel polyhydroxyalkanoates by Pseudomonas stutzeri 1317 from glucose and soybean oil. FEMS Microbiology Letters, 169, 45–49.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samrot, A.V., Avinesh, R.B., Sukeetha, S.D. et al. Accumulation of Poly[(R)-3-hydroxyalkanoates] in Enterobacter cloacae SU-1 During Growth with Two Different Carbon Sources in Batch Culture. Appl Biochem Biotechnol 163, 195–203 (2011). https://doi.org/10.1007/s12010-010-9028-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-010-9028-7