Abstract
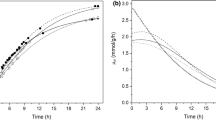
Using immobilized cells of a novel strain of Microbacterium hydrocarbonoxydans L29-9 in polymers of polyvinyl alcohol (PVA)–alginate–boric acid, enantioselective resolution of racemic γ-lactam to produce (−)γ-lactam was successfully carried out. A 6:1 ratio of PVA:sodium alginate not only prevented agglomeration of the matrix but also produced beads with high gel strength. The optimum biotransformation conditions were 1 g/L substrate, pH 7.0, reaction temperature of 30 °C, and reaction time of 3 h. After every two cycles, the immobilized cell beads were separated and immersed in 0.5 mM KCl solution at 4 °C for preservation. At optimum conditions, the enantiomeric excess and the yield of (−)γ-lactam were >99% and 34%, respectively. The beads showed a slight decrease in the enantiomeric excess when re-used up to 14 cycles (the enantioselectivity of the immobilized cells decreased slightly after 14 cycles of usage).









Similar content being viewed by others
References
Patel, R. N. (2001). Current Opinion in Biotechnology, 12, 587–604.
Weston, M. D. (2008). The comprehensive pharmacology reference. Amsterdam: Elsevier Inc.
Nakano, H., Iwasa, K., Okuyama, Y., & Hongo, H. (1996). Tetrahedron: Asymmdtry, 7, 2381–2386.
Taylor, S. J. C., Cague, R. M., Wisdom, R., Lee, C., & Dickson, K. (1993). Tetrahedron: Asymmdtry, 4, 1117–1128.
Mahmoudian, M., Lowdon, A., Jones, M., Dawson, M., & Wallis, C. (1999). Tetrahedron: Asymmdtry, 10, 1201–1206.
Taylor, S. J. C., Brown, R. C., Keene, P. A., & Taylor, I. N. (1999). Bioorganic & Medicinal Chemistry, 7, 2163–2168.
Talor, S. J. C., Sutherland, A. G., Lee, C., Wisdom, R., Thomas, S., Roberts, S. M., et al. (1990). Journal of the Chemical Society, Chemical Communications, 16, 1120–1121.
Toogood, H. S., Brown, R. C., Line, K., Keene, P. A., Taylor, S. J. C., Cague, R. M., et al. (2004). Tetrahedron, 60, 711–716.
Fernandes, P., Vidinha, P., Ferreira, T., Silvestre, H., Cabral, J. M. S., & Prazeres, D. M. F. (2002). Journal of Molecular Catalysis. B, Enzymatic, 19, 353–361.
Fatima, Y., Kansal, H., Soni, P., & Banerjee, U. C. (2007). Process Biochemistry, 42, 1412–1418.
Ellaiah, P., Prabhakar, T., Ramakrishna, B., Thaer Taleb, A., & Adinarayana, K. (2004). Process Biochemistry, 39, 525–528.
Li, G. Y., Huang, K. L., Jiang, Y. R., & Ding, P. (2007). Process Biochemistry, 42, 1465–1469.
Lozinsky, V. I., & Plieva, F. M. (1998). Enzyme and Microbial Technology, 23, 227–242.
Antczak, M. S., & Galas, E. (2001). Biomolecular Engineering, 17, 55–63.
Wu, K. Y. A., & Wisecarver, K. D. (1992). Biotechnology and Bioengineering, 39, 447–449.
Dave, R., & Madamwar, D. (2006). Process Biochemistry, 41, 951–955.
Long, Z. E., Huang, Y. H., Cai, Z. L., Cong, W., & Ouyang, F. (2004). Process Biochemistry, 39, 2129–2133.
Chen, C. S., Wu, S. H., Girdaukas, G. S., & Sih, C. J. (1982). Journal of the American Chemical Society, 104, 7294–7299.
Acknowledgements
This work was supported by the 863 program (2006AA02Z250); the 973 program (2004CB719606); the Ministry of Science and Technology, China; the Open Fund of State Key Laboratory of Microbial Resources, the Institute of Microbiology, the Chinese Academy of Sciences (SKLMR-08060).
Author information
Authors and Affiliations
Corresponding author
Additional information
Qin and Wang shared the first author of this paper.
Rights and permissions
About this article
Cite this article
Qin, X., Wang, J. & Zheng, G. Enantioselective Resolution of γ-Lactam by a Whole Cell of Microbacterium hydrocarbonoxydans (L29-9) Immobilized in Polymer of PVA–Alginate–Boric Acid. Appl Biochem Biotechnol 162, 2345–2354 (2010). https://doi.org/10.1007/s12010-010-9007-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-010-9007-z