Abstract
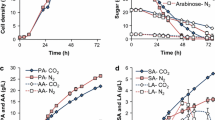
Production of succinic acid from glucose by Escherichia coli strain AFP184 was studied in a batch fermentor. The bases used for pH control included NaOH, KOH, NH4OH, and Na2CO3. The yield of succinic acid without and with carbon dioxide supplied by an adjacent ethanol fermentor using either corn or barley as feedstock was examined. The carbon dioxide gas from the ethanol fermentor was sparged directly into the liquid media in the succinic acid fermentor without any pretreatment. Without the CO2 supplement, the highest succinic acid yield was observed with Na2CO3, followed by NH4OH, and lowest with the other two bases. When the CO2 produced in the ethanol fermentation was sparged into the media in the succinic acid fermentor, no improvement of succinic acid yield was observed with Na2CO3. However, several-fold increases in succinic acid yield were observed with the other bases, with NH4OH giving the highest yield increase. The yield of succinic acid with CO2 supplement from the ethanol fermentor when NH4OH was used for pH control was equal to that obtained when Na2CO3 was used, with or without CO2 supplementation. The benefit of sparging CO2 from ethanol fermentation on the yield of succinic acid demonstrated the feasibility of integration of succinic acid fermentation with ethanol fermentation in a biorefinery for production of fuels and industrial chemicals.







Similar content being viewed by others
References
Werpy, T., & Petersen, G. (2004). Top value added chemicals from biomass, U.S. Department of Energy.
Fumagalli, C. (1997). In J. I. Kroschwitz & M. Howe-Grant (Eds.), Kirk-Othmer encyclopedia of chemical technology Vol. 22 (pp. 1072–1102). New York, NY: Wiley.
Rao, V. N. M. (1988). U.S. Patent 4,782,167.
Mabry, M. A. (1985). U.S. Patent 4,550,185.
Berglund, K. A., Dunuwila, D. D., Alizadeh, H. (2003). U. S. Patent 6,623,657.
Berglund, K. A., Dunuwila, D. D., Alizadeh, H. (2003). U.S. Patent 6,635,188.
Glassner, D. A., & Datta, R. (1992). U.S. Patent 5,143,834.
Guettler, M. V., Jain, M. K., Soni, B. K. (1996). U.S. Patent 5,504,004.
Nghiem, N. P., Donnelly, M., Millard, C. S. (1999). U.S. Patent 5,869,301.
Donnelly, M., & Nghiem, N. P. (2004). U.S. Patent 6,743,610.
Lin, H., Bennett, G. N., & San, K. Y. (2005). Biotechnology and Bioengineering, 90, 775–779.
Lee, S. J., Song, H., & Lee, S. Y. (2006). Applied and Environmental Microbiology, 72, 1939–1948.
Okino, S., Inui, M., & Yukawa, H. (2005). Applied Microbiology and Biotechnology, 68, 475–480.
Nghiem, N. P., Davison, B. H., Donnelly, M. I., Tsai, S. -P., Frye, J. G. (2000). Chemicals and materials from renewable resources. In J. J. Bozell (Ed.), American chemical society symposium series 784:160–173.
Millard, C. S., Chao, Y.-P., Liao, J. C., & Donnelly, M. I. (1996). Appl Environmental Microbiology, 62, 1808–1810.
Vemuri, G. N., Eiteman, M. A., & Altman, E. (2002). Journal of Industrial Microbiology & Biotechnology, 28, 325–332.
Anderson, C., Hodge, D., Berglund, K. A., & Rova, U. (2007). Biotechnology Progress, 23, 381–388.
Anderson, C., Helmerius, J., Hodge, D., Berglund, K. A., & Rova, U. (2009). Biotechnology Progress, 25, 116–123.
Drapcho, C. M., Nghiem, N. P., & Walker, T. H. (2008). Biofuels engineering process technology Chapter 5 (pp. 105–195). New York: McGraw-Hill.
Schill, S. R. (2008). Ethanol producer magazine, October Issue.
Gottschalk, G. (1986). Bacterial metabolism, Chapter 5 (2nd ed., pp. 127–129). New York: Springer-Verlag.
Zeikus, J. G., Jain, M. K., & Elankovan, P. (1999). Applied and Environmental Microbiology, 51, 545–552.
McKinlay, J. B., Vieille, C., & Zeikus, J. G. (2007). Applied and Environmental Microbiology, 76, 727–740.
Lu, S., Eiteman, M. A., & Altman, E. (2009). Journal of Industrial Microbiology & Biotechnology, 36, 1101–1109.
Acknowledgement
The authors would like to thank Gerard Senske and John Minutolo for skillfully performing the fermentation experiments and Michael Kurantz for the compositional analysis of the barley and corn used in this study. The many invaluable comments and suggestions of Dr. Susanne Kleff of MBI International, Lansing, MI, given during the review of the first draft of the manuscript also are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Rights and permissions
About this article
Cite this article
Nghiem, N.P., Hicks, K.B. & Johnston, D.B. Integration of Succinic Acid and Ethanol Production With Potential Application in a Corn or Barley Biorefinery. Appl Biochem Biotechnol 162, 1915–1928 (2010). https://doi.org/10.1007/s12010-010-8969-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-010-8969-1