Abstract
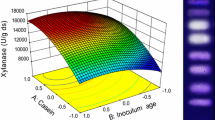
A newly isolated thermophilic fungal strain from Tunisian soil samples was identified as Talaromyces thermophilus and was selected for its ability to produce extracellular hemicellulases when grown on various lignocellulosic substrates. Following the optimization of carbon source, nitrogen source, and initial pH of the growth medium in submerged liquid cultures, yields as high as 10.00 ± 0.15 and 0.21 ± 0.02 U/ml were obtained for xylanase and β-xylosidase, respectively. In fact, wheat bran was found to be a good inducer of hemicellulase enzymes, mainly β-xylosidase. The optimal temperature and pH of the xylanase activity were 75°C and 8.0, respectively. This enzyme exhibited a remarkable stability and retained 100% of its original activity at 50°C for 7 days at pH 7.0–8.0. The half-lives of the enzyme were 4 h at 80°C, 2 h at 90°C, and 1 h at 100°C. T. thermophilus could therefore be considered as a satisfactory and promising producer of thermostable xylanases. Crude enzyme of T. thermophilus rich in xylanase and β-xylosidase was established for the hydrolysis of lignocellulosic materials as wheat bran.







Similar content being viewed by others
References
Coughlan, M. P., & Hazelwood, G. P. (1993). Biotechnology and Applied Biochemistry, 17, 259–289.
Jeffries, T. W. (1994). In C. Ratledge (Ed.), Biochemistry of microbial degradation, (pp. 233–277). Dordrecht: Kluwer.
Thomson, J. A. (1993). FEMS Microbiology Reviews, 104, 65–82.
Haltrich, D., Nidetzky, B., Kulbe, K. D., Steiner, W., & Zupancic, S. (1996). Bioressource Technology, 58, 137–161.
Polizeli, M. L. T. M., Rizzatti, A. C. S., Monti, R., Terenzi, H. F., Jorge, J. A., & Amorim, D. S. (2005). Applied Microbiology and Biotechnolology, 67, 577–591.
Techapun, C., Poosaran, N., Watanabe, M., & Sasaki, K. (2003). Process Biochemistry, 38, 1327–1340.
Hahn-Hägerdal, B., Galbe, M., Gorwa-Grauslund, M. F., Lidén, G., & Zacchi, G. (2006). Trends in Biotechnology, 24(12), 549–556.
Assamoi Allah, A., Destain, J., & Philippe, T. (2010). Applied Biochemistry and Biotechnology, 160, 50–62.
Bensod, S., Dutta-Choudhary, M., Srinivasan, C., & Rele, M. (1993). Biotechnology Letters, 15, 965–970.
Gupta, S., Kuhad, R., Bhushan, B., & Hoondal, G. (2001). World Journal Microbiology and Biotechnolology, 54, 92–97.
Ellaiah, P., Adinarayana, K., Bhavan, Y., Padmaja, P., & Srinivasulu, B. (2000). Process Biochemistry, 38, 615–620.
Galbe, M., Lidén, G., & Zacchi, G. (2005). Journal of Scientific and Industrial Research, 64, 905–919.
Kirk, O., Borchert, T. V., & Fuglsang, C. C. (2002). Current Opinion in Biotechnology, 13, 345–351.
Ines, M., Ines, B., Najla, F. M., & Belghith, H. (2009). Applied Biochemistry and Biotechnology, 158, 200–2010. doi:10.1007/s12010-008-8317-x.
Mohamed, G., Ali, G., & Hafedh, B. (2008). Applied Biochemistry and Biotechnology, 150, 267–279.
Mandels, M., & Weber, J. (1969). Advances in Chemistry Series, 95, 391–413.
Reese, E. T., & Maguire, A. (1969). Applied Microbiology, 17, 242–245.
Mandels, M., Andreotti, R., & Roche, C. (1976). Biotechnology and Bioengineering Symposium, 6, 21–34.
Miller, G. (1959). Analytical Chemistry, 31, 426–428.
Nath, R. L., & Rydon, H. (1954). In W.A. Wood, & S. T. Kellogg (Eds.), Methods in enzymology (pp. 679–684). New York: Academic.
Bradford, M. (1976). Analalytical Chemistry, 72, 248–254.
Dien, B. S., Li, X. L., Iten, L. B., Jordan, D. B., Nichols, N. N., O’Bryan, P. J., et al. (2006). Enzyme and Microbial Technology, 39, 1137–1144.
Beaugrand, J., Cronier, D., Debeire, P., & Chabbert, B. (2004). Journal of Cereal Science, 40, 223–230.
Chandra, R., & Chandra, T. (1995). Biotechnology Letters, 17, 309–314.
Royer, J., & Nakas, J. (1989). Enzyme and Microbial Technology, 11, 405–410.
Tilburn, J. (1995). European Molecular Biology Organization Journal, 14, 779–790.
Smith, D., & Wood, T. (1991). Biotechnology and Bioengineering, 38, 883–890.
Yin, L., Zhiqiang, L., Fengjie, C., Yingying, X., & Hui, Z. (2007). World Journal of Microbiology and Biotechnology, 23, 837–843.
Shah, A. R., & Madamwar, D. (2004). Process Biochemistry, 40, 1763–1771.
Simmons, E. (1977). In: Second International Mycological Congress (pp. 618–18), Tampa, Florida.
Dubeau, H., Chahal, D., & Ishaque, H. (1987). Biotechnology Letters, 4, 275–80.
Georis, J., De Lemos Esteves, F., Lamotte-Brasseur, J., Bougnet, V., Devreese, B., Giannotta, F., et al. (2000). Protein Science, 9, 466–475.
Jain, A., Garg, S. K., & Johri, B. N. (1998). Bioresource Technology, 64, 225–228.
George, S. P., Ahmad, A., & Rao, M. B. (2001). Bioresource Technology, 77, 171–175.
Kohli, U., Nigam, P., & Singh, D. (2001). Enzyme and Microbial Technology, 28, 606–610.
Gnansounou, E., Dauriat, A., & Wyman, C. E. (2005). Bioresource Technology, 96, 985–1002.
Öhgren, K., Vehmaanpera, J., Siika-Aho, M., Galbe, M., Viikari, L., & Zacchi, G. (2007). Enzyme and Microbial Technology, 40, 607–613.
Aachary, A. A., & Prapulla, S. G. (2008). Journal of Agriculture and Food Chemistry, 56(11), 981–988.
Acknowledgments
The authors wish to express their sincere gratitude to Professor Ali Gargouri for his interest in this work and for his critical appraisal of the paper. Special thanks are also due to Mr. Anouar Smaoui from the English Department at the Sfax Faculty of Science for his careful proofreading and constructive review of the manuscript of the present study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romdhane, I.B.B., Achouri, I.M. & Belghith, H. Improvement of Highly Thermostable Xylanases Production by Talaromyces thermophilus for the Agro-industrials Residue Hydrolysis. Appl Biochem Biotechnol 162, 1635–1646 (2010). https://doi.org/10.1007/s12010-010-8945-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-010-8945-9