Abstract
Fermentation of enzymatic hydrolysate of waste newspaper was investigated for cellulosic ethanol production in this study. Various nonionic and ionic surfactants were applied for waste newspaper pretreatment to increase the enzymatic digestibility. The surfactant-pretreated newspaper was enzymatically digested in 0.05 M sodium citrate buffer (pH 4.8) with varying solid content, filter paper unit loading (FPU/g newspaper), and ratio of filter paper unit/β-glucosidase unit (FPU/CBU). Newspaper pretreated with the anionic surfactant sodium dodecyl sulphate (SDS) demonstrated the highest sugar yield. The addition of Tween-80 in the enzymatic hydrolysis process enhanced the enzymatic digestibility of newspaper pretreated with all of the surfactants. Enzymatic hydrolysis of SDS-pretreated newspaper with 15% solid content, 15 FPU/g newspaper, and FPU/CBU of 1:4 resulted in a newspaper hydrolysate conditioning 29.07 g/L glucose and 4.08 g/L xylose after 72 h of incubation at 50 °C. The fermentation of the enzymatic hydrolysate with Saccharomyces cerevisiae, Pichia stipitis, and their co-culture produced 14.29, 13.45, and 14.03 g/L of ethanol, respectively. Their corresponding ethanol yields were 0.43, 0.41, and 0.42 g/g.
Similar content being viewed by others
Introduction
In view of recent developments in crude oil market prices, the use of alternative, non-petroleum-based sources of energy is expected to rise sharply in the coming years [1]. Lignocellulosic biomass such as energy crops, forest wastes, agricultural wastes, and municipal wastes could offer a huge renewable resource for second-generation biofuel production due to their abundance and low cost compared with starch- and sucrose-based materials [2, 3]. Waste paper is the most common type of waste in Singapore and about 1.26 million tonnes of waste paper was generated in 2008. The recycling rate of paper is 48% in 2008, and most of this paper waste is sorted, baled, and exported overseas for recycling as there are no paper recycling mills in Singapore [4]. Conversion of waste paper to fuel ethanol might offer a better alternative for waste paper recycling in Singapore.
Lignocelluloses are mainly comprised of cellulose, a polymer of six-carbon sugar, glucose; hemicelluloses, a branched polymer comprised of xylose and other five-carbon sugars and lignin consisting of phenyl propane units [5]. Newspaper is made up of 40–55% cellulose, 25–40% hemicelluloses, and 18–30% lignin [6]. The presence of lignin limits the fullest usage of cellulose and hemicelluloses. The lignocellulosic biomass must be pretreated to make the cellulose fraction more accessible to enzymatic hydrolysis. Diverse pretreatment processes have been evaluated technically and economically, aiming at improving enzymatic hydrolysis; these include dilute acid hydrolysis [7], alkali treatment [8], steam-explosion [9], and organic solvents [10]. However, because newspaper has already received considerable chemical and/or physical pretreatment, it does not require the extensive pretreatment as required for woody and herbaceous biomass, but a multitude of additives and inks are added during the paper producing and printing processes. A lot of work about the newspaper pretreatment to improve enzymatic hydrolysis has been reported [11, 12]. Recently, hydrogen peroxide and nonionic surfactants have been frequently used in newspaper pretreatment. Although ammonia and hydrogen peroxide can help to swell cellulosic fibers and remove inks, nonionic surfactants such as Tween and polyethylene glycol (PEG) are more preferred due to the environmental and economic aspects of these chemicals. However, to-date little research has been conducted on the comparison of pretreatment methods using a variety of ionic or nonionic surfactants.
The cellulosic and hemicellulosic sugars obtained through enzymatic hydrolysis can efficiently be used for ethanol fermentation either using pure culture or using mixed culture. However in mixed culture optimum growth conditions of the yeasts would be different and might result in lower efficiency and lower product yield [13]. Therefore for the higher yield of ethanol production, the approach of separate hydrolysis and fermentation (SHF) using pure culture was preferred.
The aim of this study is to investigate the potentials of newspaper conversion to fuel ethanol through the optimization of newspaper pretreatment, enzymatic hydrolysis, and newspaper hydrolysate fermentation. Important factors in the enzymatic hydrolysis of newspaper after a variety of ionic and nonionic surfactants pretreatment were studied. The influences of different yeast species, Saccharomyces cerevisiae, Pichia stipitis, and their co-culture in ethanol production were also discussed.
Materials and Methods
Materials
Waste newspaper was collected locally in Singapore. The cellulose content in the waste newspaper was 53.3% [14]. All chemicals were of analytical grade and obtained from Sigma–Aldrich (St. Louis, MO, USA). A commercial Trichoderma reesei cellulase (Celluclast 1.5 L) and a β–glucosidase preparation (Novozyme 188) were used in the enzymatic hydrolysis experiments. Celluclast 1.5 L and Novozyme 188 were both kindly donated by Novozymes Malaysia sdn bhd. Celluclast 1.5 L had a Filter paper (FPase) activity of 187 IU/mL and a β-glucosidase activity of 32 IU/mL. Novozyme 188 had an FPase activity of 4.7 IU/mL and a β-glucosidase activity of 795 IU/mL. Distilled water was used throughout, except for the chromatographic procedure wherein Millipore water was used.
Pretreatment of Newspaper
Pretreatment was prepared according to what described by Kim et al. [11] with some modifications. Waste newspaper was cut into approximately 0.5 × 2 cm strips. Ten grams of newspaper strips were added to a 500-mL round flask containing 200 g of deionized water. After that 0.5% (w/w) of a surfactant was added to this solution and the content of the flasks was agitated at 250 rpm and 40 °C for 1 h. The concentration of the surfactant was calculated as percentage (w/w) based on the 10 g of the dry substrate. After pretreatment, the wet solid was washed with 3 L of deionized water to completely remove the surfactants. It was then dried in an oven at 105 °C overnight. The final pretreated newspaper was then cooled down at room temperature (30 °C ± 2 °C) and stored in polyethylene bags for further use.
Enzymatic Hydrolysis
The enzymatic hydrolysis of pretreated newspaper was conducted in 50-mL Falcon tubes in 20 mL of 50 mM sodium citrate buffer (pH 4.8). The experiments were performed in duplicates at 50 ± 1 °C in a shaking water bath (Memmert GmbH + Co. KG, Schwabach, Germany) with maximum strokes for 72 h. Celluclast 1.5 L and Novozyme 188 were used throughout the hydrolysis experiments. The influence of the enzyme activities ratio of filter paper unit/β-glucosidase unit (FPU/CBU) and enzyme loading with varying FPase activity (FPU/g newspaper) were investigated. Effects of solid content were studied with the optimized enzyme loading and FPU/CBU to obtain the highest possible sugar concentration for ethanol fermentation. At the end of the hydrolysis, samples were centrifuged at 10,000×g for 10 min and analyzed for sugar concentration using the 3,5-dinitrosalicylic acid (DNS) method [15]. The sugar yields were calculated as: reducing sugar (g)×0.9×100/initial cellulose (gram) in biomass. Hydrolysis of polysaccharides involves water. For each mole of reducing sugar released, one mole of H2O is required. A correction factor of 0.9 was therefore included in the calculation of the amount of polysaccharides hydrolyzed. Experiments were carried out in duplicates.
Microorganisms and Culture Conditions
Pichia stipitis CBS 6054 was obtained from Centraalbureau voor Schimmelcultures (CBS, Baarn) Culture Collection, and it was maintained on YPX agar slants containing (grams per liter): xylose, 20.0; yeast extract, 10.0; peptone, 20.0; agar, 20.0 at pH 5.5 ± 0.2. Saccharomyces cerevisiae ATCC 96581 was procured from American Type Culture Collection (ATCC) and it was maintained on YPD agar slants containing (grams per liter): glucose, 20.0; yeast extract, 10.0; peptone, 20.0; agar, 20.0 at pH 5.5 ± 0.2. They were both incubated at 30 °C for 24 h, subsequently stored at 4 °C and were subcultured on YPX and YPD plates, respectively, at regular intervals.
The culture medium consisted of (grams per liter) yeast extracts, 10.0; urea, 6.4; KH2PO4, 2.0; MgSO4⋅7H2O, 1.0, and glucose (for S. cerevisiae), 20.0 or xylose (for P. stipitis), 20.0. The pH was adjusted to 5.5 ± 0.2 using 2 M NaOH. The newspaper enzymatic hydrolysate fermentation medium consisted of (grams per liter) glucose, 29.07; xylose, 4.08; KH2PO4, 2.0; MgSO4⋅7H2O, 1.0; yeast extracts, 10.0, and urea, 6.4. pH was adjusted to 5.5 ± 0.2 unless otherwise mentioned. The hydrolysates were autoclaved at 110 °C for 10 min while mineral salts, urea and yeast extracts were autoclaved separately at 121 °C for 30 min. They were then mixed aseptically.
Preparation of Inoculums
S. cerevisiae and P. stipitis were transferred by loop from 1-day plates to 150-mL Erlenmeyer flasks containing 50 mL of their respective culture medium. Yeasts were grown for 24 h at 150 rpm on a rotary shaker at 30 °C. Afterwards, cells were collected by centrifugation at 2,000 g and 4 °C for 15 min and were then washed in sterile distilled water. Cells were then re-suspended in 5 mL of the fermentation medium and optical density at 600 nm (OD600) was then measured to determine the cell density. One milliliter of such cell suspension was used as the inoculum for each 250-mL Erlenmeyer flask containing 100 mL of the culture medium. For the co-culture of S. cerevisiae and P. stipitis, equal amount of each yeast strain was inoculated according to their respective OD600 readings with an overall inoculum volume of 1 mL. The initial cell concentrations (dry weight) of S. cerevisiae, P. stipitis, and their co-culture in the subsequent fermentation were 2.1, 0.9, and 1.4 g/L, respectively.
Ethanol Fermentation
The enzymatic hydrolysates were fermented by S. cerevisiae, P. stipitis, and their co-culture in 250-mL Erlenmeyer flasks sealed with screwed caps containing 100 mL fermentation medium for 29 h. The shaking speed was kept at 150 rpm and temperature at 30 °C. Samples were withdrawn periodically to determine the concentration of biomass, ethanol, glycerol, xylitol, and residual sugars in the fermentation broth. Technical quality nitrogen containing less than 5 ppm O2 was flushed through the flasks while withdrawing samples to maintain an anaerobic environment. Fermentation experiments were conducted in duplicates.
Analytical Methods
Cell biomass was monitored spectrophotometrically by measuring absorbance at 600 nm. The measurement was made such that the optical density (OD600) of the samples was <0.70, as obtained by sample dilution. This is to ensure that the Beer–Lambert law applies. Forty-milliliter samples of whole culture were filtered through 0.45 μm pre-dried, pre-weighed glass fiber membrane filters and dried at 105 °C till constant weight was obtained. Biomass dry weight was calculated as the difference between the membrane dry weight before and after the culture broth filtration. A calibration curve was prepared between OD600 and biomass dry weight. The OD value was then converted to biomass dry weight using such calibration curve. Biomass concentration (grams per liter) was found to follow the regression equation: \( X\left( {{\text{g}}/{\text{L}}} \right) = 0.{\text{314}}\left( {{\text{O}}{{\text{D}}_{{\text{6}}00}}} \right) \).
The amount of reducing sugars released from enzymatic hydrolysis was measured using the DNS method [15]. Concentration of individual sugars and their metabolites were measured using high-performance liquid chromatography (HPLC). Samples were filtered through 0.45 µm filters and stored at −20 °C until analyzed by a 1200 Series HPLC system (Agilent Technologies Inc.) equipped with a Refractive Index Detector. Glucose, xylose, ethanol, acetic acid, glycerol, and xylitol were analysed on an Aminex HPX-87H column (Bio-Rad, Richmond, CA, USA) at 75 °C with 0.6 mL/min eluent of 5 mM sulfuric acid.
Results and Discussion
Effect of Different Surfactants on Newspaper Pretreatment
A number of pretreatments such as electron beam irradiation [16], carbon dioxide explosion [17], nonionic surfactants [18], steam explosion [19], and ammonia-hydrogen peroxide [20] have been used to improve the saccharification efficiency of the newspaper. Among these methods, nonionic surfactants pretreatment for enzymatic hydrolysis has been most popular not only because it requires low energy and/or low chemical cost, but also because these surfactants increase cellulase activity during enzymatic hydrolysis and they do not inhibit cell growth during subsequent fermentation processes. It is believed that surfactants efficiently help remove the ink and other components that physically interfere with enzymatic hydrolysis. Kim et al. [21] found that pretreatment with Tween-80 alone was sufficient to improve the newspaper digestibility and addition of other chemicals such as ammonia, hydrogen peroxide, and their combination with Tween-80 did not help much in the improvement of newspaper digestibility. This revealed that newspaper should not be pretreated using the methods developed for wood and herbaceous biomass.
To investigate the effect of the addition of different surfactants in the newspaper pretreatment process, raw newspaper strips was pretreated using water and various surfactants at a concentration of 0.5% (w/w) to determine their pretreatment performance. The digestibility of the pretreated newspaper was measured through enzymatic saccharification using the mixture of commercial enzymes, Celluclast 1.5 L and Novozyme 188. The untreated newspaper was used as the control. The experiments were conducted in duplicates at 50 °C in 0.05 M citrate buffer at pH 4.8 for 72 h with 0.5% solid content, an enzyme loading of 5 FPU/g pretreated newspapers, and an enzyme ratio of Celluclast 1.5 L FPU/Novozyme 188 CBU of 1:1. Sugar yield was calculated for each experiment. As shown in Fig. 1, compared with the control, the pretreatment using SDS showed the highest sugar yield. SDS was used in the hydrolysis of steam-pretreated spruce [22]. While SDS was most efficient in decreasing the enzyme adsorption it did not improve the biomass digestibility effectively as SDS denatured the enzymes. Pretreatment of newspaper using SDS is the first report and it seemed to be the most efficient pretreatment reagent compared with the nonionic surfactants used. Both nonionic and ionic surfactants are being used in the de-inking process of waste paper. Surfactants are effective in improving the wetting of ink particles by lowering the surface tension of the water and therefore help the detachment of ink particles from the newspaper. In addition, surfactants can serve as dispersants to dissolve the detached ink particles and create a stable emulsion that does not readily deposit in the newspaper fiber. In the latter case, the anionic surfactants are more efficient than the nonionic surfactants as both the newspaper fiber surface and the ink emulsion are negatively charged. This prevents the redeposition of ink particles on the surface of the paper pulp [23, 24]. This agrees quite well with our findings that the SDS-pretreated newspaper was the best in brightness and in enzyme digestibility. Among all the non-ionic surfactants investigated, pretreatment with Triton X-100 showed the highest enzyme digestibility, with the rest nonionic surfactants showing slight improvements in the enzyme digestibility of the newspaper compared with the control. This is consistent with what reported in the literature [21]. However, the enhancement of biomass digestibility using surfactant pretreatment is sensitive to substrate type, biomass composition, biomass structure, enzyme loading, surfactant's structure, and molecular weight [11, 21]. The combination of these factors may contribute to the slight differences in the enzyme digestibility of the newspaper treated with various surfactants. It is worth noting that the enzyme digestibility was very sensitive to the amount of residual SDS in the SDS-pretreated newspaper. The complete removal of the residual SDS was very essential in the enhancement of enzyme digestibility of newspaper. Otherwise, the residual SDS might influence the newspaper hydrolysis efficiency negatively. The full removal of residual SDS was indicated by the fact that no bubbles were observed in the final washing solution from the treated newspaper.
Effect of different pretreatment methods using different ionic and nonionic surfactants on the sugar yield of newspaper and the effect of Tween-80 on the enzymatic hydrolysis of pretreated newspaper at 50 °C, 5% solid content, 5 FPU/g newspaper and FPU/CBU of 1:1 after 72 h. (T Tween, SDS sodium dodecyl sulphite, TX Triton X, P polyethylene glycol)
Effect of Tween-80 on Enzymatic Hydrolysis
The positive effect of surfactants on enzymatic hydrolysis has been reported a number of times [22, 25–27]. The digestibility of newspaper was previously reported to dramatically increase when Tween-80 or other surfactants were added at the enzymatic hydrolysis stage [28]. Kim et al. reported that Tween-80 could slightly enhance the enzyme reaction when using nonionic surfactant pretreated newspaper and the surfactant loading had almost no effect on digestibility over 0.5% [11]. The mechanism behind it was reported to be the enhancement of enzyme stability and the prevention of non-specific binding between the enzymes and lignin [22].
To investigate the effect of Tween-80 on the hydrolysis, various pretreated newspapers were hydrolyzed in the presence of 0.5% (v/v) Tween-80. Newspaper strip without any chemical pretreatment was chosen as the control. Sugar yield results obtained from enzymatic hydrolysis experiments with and without Tween-80 are displayed in Fig. 1. It can be seen that the addition of Tween-80 generally enhanced the enzyme accessibility of all the pretreated newspaper and the highest improvement was obtained for the SDS-pretreated newspaper. This concurs with what has been reported that the addition Tween-series surfactants could enhance the enzymatic hydrolysis of biomass [21]. It was interesting to note that in the presence of Tween-80 the final sugar yields obtained from the newspapers pretreated with water, Tween-80, Triton X-100, and PEG-10000 were almost identical. This implies that the enhancement of enzyme digestibility of newspaper by the addition of Tween-80 might have reached its potential, whereas, on the other hand, ink removal efficiency is the most important factor that contributes to the further enhancement of enzyme digestibility of the pretreated newspaper. As newspaper pretreated with SDS alone without the addition of Tween-80 in the hydrolysis process gave higher sugar yield compared with those treated with the nonionic surfactants with the addition of Tween-80 during hydrolysis, SDS pretreatment in the absence of surfactant during the hydrolysis process was applied through the rest of the experiments.
Effect of Cellulase Loading and Ratio of FPU/CBU on Enzymatic Hydrolysis
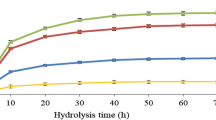
Cellulase dosage of 10 FPU/g cellulose is often used in laboratory studies because it provides a hydrolysis profile with high levels of glucose yield in a reasonable time (48–72 h) at a reasonable enzyme cost. Cellulase enzyme loadings in hydrolysis vary from 7 to 33 FPU/g substrate, depending on the types and concentrations of substrates [29]. The effect of cellulase loading (3 ∼ 25 FPU/g newspaper) on the sugar yield is shown in Fig. 2. As expected, sugar yield increased with increasing cellulase loading from 3 to 15 FPU/g newspaper. A 54.9% sugar yield was obtained when cellulase loading was 15 FPU/g newspaper. An increase of 45% of sugar yield was obtained at this condition compared with that obtained with 3 FPU/g. However, when the cellulose loading was increased further, the sugar yield decreased slightly with the increase of cellulase dosage. This might be due to the inhibitory effects resulted from the accumulation of end-products such as glucose and cellobiose obtained at higher cellulase concentration or due to the insufficient contact of the enzymes and the substrate under such condition. As such, cellulase loading of 15 FPU/g newspaper was considered as the optimum value and it was adopted throughout the rest of the experiments.
The commercial Celluclast 1.5 L is obtained aerobically from T. reesei, which releases a mixture of cellulases, among which cellobiohydrolases, endoglucanases, β-glucosidases, and hemicellulases can be found [30]. The action of cellobiohydrolases causes a gradual decrease in the polymerization degree while endoglucanases cause the rupture of cellulose in smaller chains reducing rapidly the polymerization degree [31]. Although Celluclast 1.5 L contains some β-glucosidases (32 IU/ml), which are responsible of hydrolyzing cellobiose into glucose, their activities are rather low. Unfortunately, cellobiohydrolases are inhibited by the cellobiose released. Therefore the presence of a sufficient amount of β-glucosidase is important in order to complement the action of the cellulases [32]. To increase the sugar yield, higher concentrations of cellulases are required (Fig. 2), meanwhile the proper ratio of cellulase unit and beta-glucosidase unit (FPU/CBU) is necessary. The impact of varying β-glucosidase supplementation on sugar yield was determined with the fixed Celluclast 1.5 L dosage of 15 PFU/g newspaper. The results are displayed in Fig. 3. Sugar yield increased markedly with the increase of β-glucosidase supplementation, indicating that alleviation of inhibition from cellobiose is very critical for the efficient biomass enzymatic hydrolysis. Sugar yield reached a maximum value of 98.6% when the ratio of FPU/CBU was 1:4. This value almost doubled the sugar yield obtained from the control where no β-glucosidase was supplemented. When the amount of β-glucosidase supplemented increased further, a decrease in the sugar yield was observed. Like the case of cellulase loading, it might be due to the inhibitory effects of the end-product, glucose. In addition, insufficient interaction between the substrate and the enzymes might also contribute to the decreased sugar yield under higher enzyme concentrations. The optimal ratio of FPU/CBU was therefore 1:4 for the hydrolysis of SDS-pretreated newspaper. This value was adopted for the rest of experiments.
Effect of Solid Content on Enzymatic Hydrolysis
Fermentation at high solid content is an important parameter in enhancing the economic attractiveness of processing waste newspaper into value-added products. Substrate concentration is one of the main factors that affect the yield and the initial rate of enzymatic hydrolysis of cellulose. At low substrate levels, an increase of substrate concentration normally results in an increase of the sugar yield and reaction rate of the hydrolysis [32]. However, high substrate concentration can cause substrate inhibition, which substantially lowers the rate of the hydrolysis, and the extent of substrate inhibition depends on the ratio of total substrate to total enzymes [33, 34]. During industrial operations, higher biomass loadings of 15% (w/w) or higher can result in higher sugar concentrations and in turn greater ethanol titers while minimizing the fermenter volume required in spite of material handling difficulties [35]. Therefore experiments were conducted to determine the impact of varying the solid content (w/w) from 5% to 17.5% on glucose concentration.
Figure 4 displays the impact of varying solid contents on glucose concentration in enzymatic hydrolysis of SDS-pretreated newspaper. The cellulase dosage was 15 FPU/g newspaper and the enzyme ratio (FPU/CBU) was 1:4. Experiments were conducted at 50 °C for 72 h. Reducing sugar concentration increased with the increase of newspaper concentration and it reached a plateau when the solid content was 15% (w/w). The maximum sugar concentration obtained was 32.4 g/L measured by using the DNS method [15]. Further increase in the solid content did not result in any increase in sugar concentration. This might be due to the saturation of the enzyme active sites or be attributed to the insufficient mass transfer caused by the high biomass loading. Furthermore, higher substrate concentration may cause end-products (glucose and cellobiose) inhibition. This substantially lowers the rate of the hydrolysis. The extent of substrate inhibition depends on the ratio of total substrate concentration to total enzyme loading. The results listed here suggest that the highest sugar concentration can be obtained from the SDS-pretreated newspaper using the optimized condition in this study was 32.4 g/L although higher sugar concentration was reported from other lignocellulosic biomass [35, 36]. This might be due to the much lower density of newspaper compared with other lignocellulosic biomass.
Fermentation of Newspaper Hydrolysates
The fermentation profile of enzymatic hydrolysates containing 29.07 g/L glucose and 4.08 g/L xylose using S. cerevisiae, P. stipitis, and their co-culture are depicted in Figs. 5, 6, and 7, respectively. The maximum ethanol concentration, ethanol yield, its corresponding ethanol productivity, specific productivity, biomass concentration, glycerol concentration, and time of fermentation are compared in Table 1. Several evidences suggest that separate fermentation by substrate-specific organisms works better instead of using mixed culture [37]. Mixed culture fermentation associates both hexose and pentose fermenting yeasts that trade-off for oxygen requirement between the two microorganisms.
S. cerevisiae, which is unable to utilize either xylose or arabinose, is the favored microorganism for converting glucose into ethanol. Fermentation of newspaper hydrolysate using S. cerevisiae resulted the fastest rate of biomass growth and ethanol production. Maximum ethanol concentration of 14.29 g/L was obtained at 8 h with an ethanol yield of 0.43 g/g and an ethanol productivity of 1.79 g−1 L−1 h−1. As the initial biomass concentration of fermentation for each culture was different for this study, the specific ethanol productivity was calculated for all three culture and the results are listed in Table 1. A specific productivity of 0.75 g−1 L−1 h−1 g−1 biomass was obtained for the fermentation using S. cerevisiae alone. Glucose was consumed rapidly whereas xylose was consumed slightly. Compared with S. cerevisiae, P. stipitis grew much more slowly. Maximum concentration of ethanol (13.45 g/L) was obtained at 29 h, with an ethanol yield of 0.41 g/g and an ethanol productivity of 0.46 g−1 L−1 h−1 at that time. Compared with fermentation using S. cerevisiae, much lower specific ethanol productivity, 0.17 g−1 L−1 h−1 g−1 biomass was obtained for fermentation using P. stipitis alone. Glucose consumption in this case was slower than that using S. cerevisiae alone and xylose consumption was still insignificant. Although P. stipitis could ferment xylose, the presence of glucose might inhibit xylose uptake in P. stipitis [38]. Xylose consumption started slowly after glucose was fully consumed as can be seen in Fig. 6. Co-culture of S. cerevisiae and P. stipitis did not result in much improvement in xylose utilization as can be seen from Fig. 7. However, compared with fermentation using P. stipitis alone, both rates for biomass growth and ethanol production were improved. This may be due to the complementary effects from S. cerevisiae in glucose uptake and in turn glucose utilization. The maximum ethanol concentration of 14.03 g/L was obtained at 11 h with an ethanol yield of 0.42 g/g and an ethanol productivity of 1.28 g−1 L−1 h−1. The ethanol-specific productivity in this case was 0.59 g−1 L−1 h−1 g−1 biomass, much better than that obtained from fermentation using P. stipitis alone. However, fermentation using S. cerevisiae alone still gave the highest ethanol concentration, ethanol yield, ethanol productivity, and ethanol-specific productivity. The ethanol yields obtained in this study using S. cerevisiae alone (0.43 g/g), P. stipitis alone (0.41 g/g) and co-culture (0.42 g/g) were very much in agreement with the reported results of ethanol yield 0.40 g/g from sweet sorghum [39], and 0.48 g/g from corncobs [40]. Fermentation using S. cerevisiae alone gave the highest ethanol yield and co-cultivation of P. stipitis with S. cerevisiae did not improve the ethanol yield. This suggests that when xylose concentration is low in the sugar mixture, using S. cerevisiae alone is more advantageous.
In this study, glycerol was the main by-product; however its concentration was rather low in all the three cases though fermentation using P. stipitis alone showed the lowest glycerol production (Table 1). Xylitol was another by-product. Under the conditions investigated, its production was pretty low (less 0.01 g/L) for both fermentation using S. cerevisiae and the co-culture of S. cerevisiae and P. stipitis. No xylitol was detected at all in the fermentation using P. stipitis alone. The results reported here indicate that the detected xylitol was produced by S. cerevisiae through the utilization of xylose. S. cerevisiae has been reported to have endogenous genes encoding xylose reeducates (XR) and xylitol dehydrogenate (XDH) activities [41, 42]. This allows the growth of S. cerevisiae on xylose and the accumulation of xylitol was due to the imbalance in the co-factors for XR and XDH in S. cerevisiae. However both XR and XDH activities in S. cerevisiae are very low, its consumption of xylose is therefore very limited. It is worth noting that in the co-culture of S. cerevisiae and P. stipitis, diauxic growth was observed for both biomass growth and ethanol production at 6 h. Glucose concentration reached to a constant at that point followed by a sharp decrease. Meanwhile xylose consumption rate increased slightly after that time. This implies that P. stipitis starts to consume the substrates at a later time than S. cerevisiae. The increased ethanol and biomass production rate after that time was attributed to the simultaneous growth and metabolic activities of both yeast strains.
Conclusion
The current work demonstrated that waste newspaper was a potential substrate for cellulosic ethanol production. Anionic surfactant SDS can be effectively used for waste newspaper pretreatment to enhance its enzymatic digestibility. Newspaper hydrolysis conducted with a solid content of 15%, a filter paper cellulase loading of 15 FPU/g newspaper and an enzyme ratio FPU/CBU of 1:4 resulted in a newspaper hydrolysate with 29.07 g/L of glucose and 4.08 g/L of xylose. Fermentation of such biomass hydrolysate using S. cerevisiae and P. stipitis revealed that fermentation using S. cerevisiae was most effective. It showed the highest ethanol yield (0.43 g/g) and highest productivity (1.79 g−1 L−1 h−1). Fermentation using P. stipitis alone, on the other hand, demonstrated the lowest productivity (0.46 g−1 L−1 h−1) to obtain the comparable ethanol yield (0.41 g/g). Co-culture of S. cerevisiae and P. stipitis did not show any advantages over fermentation using S. cerevisiae alone though a comparable ethanol yield of 0.42 g/g and a much improved ethanol productivity of 1.28 g−1 L−1 h−1 were obtained compared with the fermentation using P. stipitis alone. The results presented here suggest that S. cerevisiae is a good ethanol producer when the amount of xylose in the biomass hydrolysate is insignificant.
References
Kumar, R., Singh, S., & Singh, O. V. (2008). Journal of Industrial microbiology and Biotechnology, 35, 377–391.
Hahn-Hägerdal, B., Galbe, M., Gorwa-Grauslund, M. F., Lidén, G., & Zacchi, G. (2006). Trends in Biotechnology, 24, 549–556.
Tengerdy, R. P., & Szakacs, G. (2003). Biochemical Engineering Journal, 13, 169–179.
Paper Recycling 2008. Available from http://www.zerowastesg.com/2008/12/08/paper-recycling/, Accessed Aug 6, 2009.
Zaldivar, J., Nielsen, J., & Olsson, L. (2001). Applied Microbiology and Biotechnology, 56, 17–34.
Sun, Y., & Cheng, J. (2002). Bioresource Technology, 83, 1–11.
Cara, C., Ruiz, E., Oliva, J. M., Saez, F., & Castro, E. (2008). Bioresource Technology, 99, 1869–1876.
Carrillo, F., Lis, M. J., Colom, X., Valldeperas, M., & Valldeperas, J. (2005). Process Biochemistry, 40, 3360–3364.
Ohgren, K., Galbe, M., & Zacchi, G. (2005). Applied Biochemistry and Biotechnology, 124, 1055–1067.
Xu, F., Sun, J. X., Liu, C. F., & Sun, R. C. (2006). Carbohydrate Research, 341, 253–261.
Kim, H. J., Kim, S. B., & Kim, C. J. (2007). Biotechnology and Bioprocess Engineering, 12, 147–151.
Kim, S. B., & Chun, J. W. (2004). Applied Biochemistry and Biotechnology, 12, 113–116.
Olsson, L., & Hahn-Hagerdal, B. (1993). Process Biochemistry, 28, 249–257.
Xin, F., & Geng, A. L. (2009). Applied Biochemistry and Biotechnology, doi:10.1007/s12010-009-8745-2. published on-line.
Miller, G. L. (1959). Analytical Chemistry, 31, 426–428.
Khan, A. W., Labrie, J., & McKeown, J. (1987). Radiation Physics and Chemistry, 29, 117–120.
Zheng, Y., Lin, H., & Tsao, G. T. (1998). Biotechnology Progress, 14, 890–896.
Park, J. W., Takahata, Y., Kajiuchi, T., & Akehata, T. (1992). Biotechnology and Bioengineering, 39, 117–120.
Cantarell, M., Cantarella, L., Gallifuoco, A., Spera, A., & Alfani, F. (2004). Process Biochemistry, 39, 1533–1542.
Kim, S. B., & Moon, N. K. (2007). Applied Biochemistry and Biotechnology, 106, 365–373.
Kim, S. B., Kim, H. J., & Kim, C. J. (2006). Applied Biochemistry and Biotechnology, 129–132, 486–495.
Eriksson, T., Börjesson, J., & Tjerneld, F. (2002). Enzyme and Microbial Technology, 31, 353–364.
Letscher, M. B. (1992). US5281438.
Zhao, Y. L., Deng, Y. L., & Zhu, J. Y. (2004). Progress in Paper Recycling, 14, 41–45.
Kristensen, J. B., B'orjesson, J., Bruun, M. H., Tjerneld, F., & Jørgensen, H. (2007). Enzyme and Microbial Technology, 40, 888–895.
Ooshima, H., Sakata, M., & Harano, Y. (1986). Biotechnology and Bioengineering, 28, 1727–1734.
Helle, S. S., Duff, S. J. B., & Cooper, D. G. (1993). Biotechnology and Bioengineering, 42, 611–617.
Castanon, M., & Wilke, C. R. (1981). Biotechnology and Bioengineering, 23, 1365–1372.
Gregg, D. J., & Saddler, J. N. (1996). Biotechnology and Bioengineering, 51, 375–383.
Beldman, G., Rombouts, F. M., Voragen, A. G. J., & Pilnik, W. (1984). Enzyme and Microbial Technology, 6, 503–507.
Ghose, T. K., & Bisaria, V. S. (1979). Biotechnology and Bioengineering, 21, 131–146.
Cheung, S. W., & Anderson, B. C. (1997). Bioresource Technology, 59, 81–96.
Huang, X. L., & Penner, M. H. (1991). Journal of Agricultural and Food Chemistry, 39, 2096–2100.
Himmel, M. E., Baker, J. O., Overend, R. P., Penner, M. H., & Liaw, E.-T. (1994). Enzymatic conversion of biomass for fuels production (pp. 363–371). Washington, DC: American Chemical Society.
Kim, Y., Hendrickson, R., Mosier, N. S., Ladisch, M. R., Bals, B., Balan, V., et al. (2008). Bioresource Technology, 99, 5206–5215.
Zhong, C., Lau, M. W., Balan, V., Dale, B. E., & Yuan, Y. J. (2009). Applied Microbiology and Biotechnology, doi:10.1007/s00253-009-2001-0.
Delgenes, J. P., Moletta, R., & Navarro, J. M. (1996). Enzyme and Microbial Technology, 19, 220–225.
Kilian, S. G., & van Uden, N. (1988). Applied Microbiology and Biotechnology, 27, 545–548.
Mamma, D., Christakopoulos, P., Koullas, D., Kekos, D., Macris, B. J., & Koukios, E. (1995). Biomass and Bioenergy, 8, 99–103.
Chen, M., Xia, L., & Xue, P. (2007). International Biodeterioration and Biodegradation, 59, 85–89.
Richard, P., Toivari, M. H., & Penttila, M. (1999). FEBS Letters, 457, 135–138.
Traff-Bjerre, K. L., Jeppsson, M., Hahn-Hagerdal, B., & Gorwa-Grauslund, M. F. (2004). Yeast, 21, 141–150.
Acknowledgements
The authors are grateful for the financial support to this work from Singapore Totalisation Board and Ngee Ann Kongsi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xin, F., Geng, A., Chen, M.L. et al. Enzymatic Hydrolysis of Sodium Dodecyl Sulphate (SDS)—Pretreated Newspaper for Cellulosic Ethanol Production by Saccharomyces cerevisiae and Pichia stipitis . Appl Biochem Biotechnol 162, 1052–1064 (2010). https://doi.org/10.1007/s12010-009-8861-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8861-z