Abstract
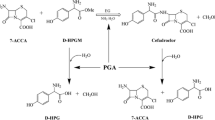
A cascade reaction combining the enzymatic hydrolysis of Penicillin G potassium salt (PGK) with the kinetically controlled enzymatic coupling of in situ formed 6-aminopenicillanic acid (6-APA) with p-hydroxyphenylglycine methyl ester (D-HPGM) to give amoxicillin as the final product by using a single enzyme has been demonstrated successfully. Ethylene glycol (EG) was employed as a component of reaction buffer to improve the synthesis yield. Reaction parameters, including different cosolvents, EG content, the loading of immobilized penicillin G acylase (IPA), and reaction temperature and time were studied to evaluate their effects on the reaction. The best result of 55.2% yield was obtained from the reaction which was carried out in the mixed media containing 40% sodium dihydrogen phosphate buffer (apparent pH 6.0) and 60% EG (v/v), with the initial concentration 150 mM and 450 mM of PGK and D-HPGM, respectively, catalyzed by 50 IU/mL IPA at 25 °C for 10 h. The IPA could be recycled for nine batches without obviously losing of catalytic activity. The important strategy will have potential application in the β-lactam antibiotics industry due to the advantages of saving the effort of isolating 6-APA, reducing usual enzymatic steps and the industrial cost of amoxicillin synthesis.







Similar content being viewed by others
Abbreviations
- PGK:
-
Penicillin G potassium salt
- 6-APA:
-
6-Aminopenicillanic acid
- D-HPGM:
-
p-Hydroxyphenylglycine methyl ester
- EG:
-
Ethylene glycol
- IPA:
-
Immobilized penicillin G acylase
- PGA:
-
Penicillin G acylase
- CLEAs:
-
Cross-linked penicillin acylase aggregates
- 7-ADCA:
-
7-Aminodeacetoxycephalosporanic Acid
- HPGA:
-
p-Hydroxyphenylglycine amide
- HPLC:
-
High-performance liquid chromatography
- UV:
-
Ultraviolet
References
Bruggink, A., Roos, E. C., & de Vroom, E. (1998). Organic Process Research and Development, 2, 128–133.
Wegman, M. A., Jassen, M. H. A., van Rantwijk, F., & Sheldon, R. A. (2001). Advanced Synthesis and Catalysis, 343, 559–576.
Diender, M. B., Straathof, A. J. J., van de Dose, T., Zomerdijk, M., & Heijnen, J. J. (2000). Enzyme and Microbial Technology, 27, 576–582.
Van Langen, L. M., De Vroom, E., Van Ranatwijk, F., & Sheldon, R. (1999). FEBS Letter, 456, 89–92.
Youshko, M. I., Moody, H. M., Bukhanov, A. L., Boosten, W. H. J., & Svedas, V. K. (2004). Biotechnology and Bioengineering, 85, 323–329.
Illanes, A., Wilson, L., Caballero, E., Fernandez-Lafuente, R., & Guisan, J. M. (2006). Applied Biochemistry and Biotechnology, 133, 189–202.
Chow, Y., Li, R., Wu, J., Puah, S. M., New, S. W., Chia, W. Q., et al. (2007). Biotechnology and Bioprocess Engineering, 12, 390–398.
Chen, C. X., Wu, Q., Liu, B. K., Lv, D. S., & Lin, X. F. (2008). Enzyme and Microbial Technology, 42, 601–607.
Spiess, A. C., & Kasche, V. (2001). In A. van Broekhoven (Ed.), Novel frontiers in the production of compounds for biomedical use (pp. 169–192). Amsterdam: Kluwer.
Ulijn, R. V., De Martin, L., Halling, P. J., Moore, B. D., & Janssen, A. E. M. (2002). Journal of Biotechnology, 99, 215–222.
Goncalves, L. R. B., Giordano, R. L. C., & Giordano, R. C. (2005). Process Biochemistry, 40, 247–256.
Goncalves, L. R. B., Sousa, R., Fernandez-Lafuente, R., Guisan, J. M., Giodano, R. L. C., & Giordano, R. C. (2002). Biotechnology and Bioengineering, 80, 622–631.
Goncalves, L. R. B., Fernandez-Lafuente, R., Guisan, J. M., & Giordano, R. L. C. (2000). Applied Biochemistry and Biotechnology, 84–86, 931–945.
Goncalves, L. R. B., Fernandez-Lafuente, R., Guisan, J. M., Giordano, R. L. C., & Giordano, R. C. (2003). Biotechnology and Applied Biochemistry, 38, 77–85.
Silva, J. A., Neto, E. H. C., Adriano, W. S., Ferreira, A. L. O., & Goncalves, L. R. B. (2008). World Journal of Microbiology & Biotechnology, 24, 1761–1767.
Chow, Y., Wu, J. C., & Li, R. J. (2005). Biocatalysis and Biotransformation, 23, 347–351.
Justiz, O. H., Fernandez-Lafuente, R., Guisan, J. M., Negri, P., Pagani, G., Pregnolato, M., et al. (1997). Journal of Organic Chemistry, 62, 9099–9106.
Schroen, C. G. P. H., Nierstrasz, V. A., Bosma, R., Kroon, P. J., Tjeerdsma, P. S., DeVroom, E., et al. (2002). Biotechnology and Bioengineering, 80, 144–155.
Wegman, M. A., van Langen, L. M., van Rantwijk, F., & Sheldon, R. A. (2002). Biotechnology and Bioengineering, 79, 356–361.
Done, S. H., Brannigan, J. A., Moody, P. C. E., & Hubbard, R. E. (1998). Journal of Molecular Biology, 284, 463–475.
Pan, S. B., Wu, Q., Chen, C. X., & Lin, X. F. (2008). Journal of Molecular Catalysis. B, Enzymatic, 54, 13–18.
Carrea, G. & Riva, S. (2008). Organic synthesis with enzymes in non-aqueous media. KGaA, Weinheim: Wiley-VCH Verlag GmbH & Co.
Fernandez-Lafuente, R., Rosell, C. M., & Guisan, J. M. (1996). Biotechnology and Applied Biochemistry, 24, 139–143.
Fernandez-Lafuente, R., Rosell, C. M., & Guisan, J. M. (1998). Enzyme and Microbial Technology, 23, 305–310.
Kim, M. G. & Lee, S. B. (1996). Journal of Molecular Catalysis. B, Enzymatic, 1, 201–211.
Park, C. B., Lee, S. B., & Dewey, D. Y. R. (2000). Journal of Molecular Catalysis. B, Enzymatic, 9, 275–281.
Kim, M. G., & Lee, S. B. (1996). Journal of Molecular Catalysis. B, Enzymatic, 1, 181–190.
Illanes, A., & Fajardo, A. (2001). Journal of Molecular Catalysis. B, Enzymatic, 11, 587–595.
Illanes, A., Anjarí, S., Arrieta, R., & Aguirre, C. (2002). Applied Biochemistry and Biotechnology, 97, 165–180.
Aguirre, C., Opazo, P., Venegas, M., Riberos, R., & Illanes, A. (2006). Process Biochemistry, 41, 1924–1931.
Wei, D. Z., & Yang, L. (2003). Journal of Chemical Technology and Biotechnology, 78, 431–436.
Ferreira, J. S., Straathof, A. J. J., Franco, T. T., & Vander Wielen, L. A. M. (2004). Journal of Molecular Catalysis. B, Enzymatic, 27, 29–35.
Acknowledgments
The financial supports from National Natural Science Foundation of China (No. 20704037) and Zhejiang Provincial Science and Technology Council (No. 2005C11023-03) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Q., Chen, CX., Du, LL. et al. Enzymatic Synthesis of Amoxicillin via a One-pot Enzymatic Hydrolysis and Condensation Cascade Process in the Presence of Organic Co-solvents. Appl Biochem Biotechnol 160, 2026–2035 (2010). https://doi.org/10.1007/s12010-009-8847-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8847-x