Abstract
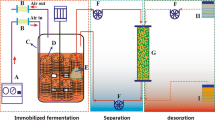
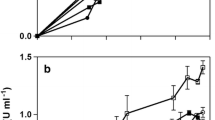
The production of extracellular and mycelia-associated penicillin G acylase (maPGA) with Mucor griseocyanus H/55.1.1 by surface-adhesion fermentation using Opuntia imbricata, a cactus, as a natural immobilization support was studied. Enzyme activity to form 6-aminopencillanic acid (6-APA) from penicillin G was assayed spectrophotometrically. The penicillin G hydrolysis to 6-APA was evaluated at six different times using PGA samples recovered from the skim milk medium at five different incubation times. Additionally, the effect of varying the penicillin G substrate concentration level on the PGA enzyme activity was also studied. The maximum reaction rate, V max, and the Michaelis constant, K M, were determined using the Michaelis–Menten model. The maximum levels for maPGA and extracellular activity were found to be 2,126.50 international unit per liter (IU/l; equal to 997.83 IU/g of support) at 48 h and 755.33 IU/l at 60 h, respectively. Kinetics of biomass production for total biomass showed a maximum growth at 60 h of 3.36 and 2.55 g/l (equal to 0.012 g of biomass per gram of support) for the immobilized M. griseocyanus biomass. The maPGA was employed for the hydrolysis of penicillin G to obtain 6-APA in a batch reactor. The highest quantity of 6-APA obtained was 226.16 mg/l after 40-min reaction. The effect of substrate concentration on maPGA activity was evaluated at different concentrations of penicillin G (0–10 mM). K M and V max were determined to be 3.0 × 10−3 M and 4.4 × 10−3 mM/min, respectively.





Similar content being viewed by others
References
Thykaer, J., & Nielsen, J. (2003). Metab Eng, 5, 56–69.
Shewale, J. G., & Sivaraman, H. (1989). Process Biochem, 24, 146–154.
Souza, V. R., Silva, A. C. G., Pinotti, L. M., Selistre-Araújo, H. S., & Camargo-Giordano, R. L. J. (2005). Braz Arch Biol Technol, 48, 105–111.
Chisti, Y., & Moo-Young, M. (1991). In V. Moses & R. E. Cape (Eds.), Biotechnology: The science and the business (pp. 167–209). New York: Harwood.
Savidge, T. A. (1984). In E. J. Vandamme (Ed.), Biotechnology of industrial antibiotics-drugs and pharmaceutical sciences (Vol. 2, pp. 172–224). New York: Dekker.
Shewale, J. G., Deshpande, B. S., Sudhakaran, V. K., & Ambedkar, S. S. (1990). Process Biochem, 25, 97–103.
Matsumoto, K. (1993). In A. Tanaka, T. Tosa & T. Kobayashi (Eds.), Production of 6-APA, 7-ACA and 7-ADCA by immobilized penicillin and cephalosporin amidases (pp. 67–88). New York: Dekker.
Martínez Hernández, J. L., Ilyina, A., Domínguez Malfavón, L., & Dustet, M. J. C. (2003). Vestn Mosk U KHIM, 44, 53–56.
Huber, F. M., Chauvette, R. R., & Jackson, B. G. (1972). In E. H. Flynn (Ed.), Preparative methods for 7-aminocephalosporanic acid and 6-aminopenicillanic acid, cephalosporins and penicillins (pp. 27–33). New York: Academic.
Vandamme, E. J. (1988). In M. Moo-Young (Ed.), Bioreactor immobilized enzymes and cells: fundamentals and applications (pp. 261–268). New York: Elsevier.
Kallenberg, A. I., van Rantwijk, F., & Sheldon, R. A. (2005). Adv Synth Catal, 347, 905–926.
Iqbal, M., & Saeed, A. (2005). Lett Appl Microbiol, 40, 178–182.
Becerra-Jiménez, J. M. E., Martínez-Hernández, J. L., Rodríguez-Martínez, J., & Ilyina, A. (2006). Mosc Univ Chem Bull, 61, 36–43.
Ilyina, A., Huerta-Guel, P. J., Martínez-Hernández, J. L., Rodríguez-Martínez, J., & Gorokhovsky, A. (2008). J Mol Catal B Enzym, 51, 1–9.
Raimbault, M. (1998). Elect J Biotech, 1, 1–15. available from http://www.ejb.org. Accessed June 10, 2009.
Aguilar, C. N., Gutiérrez-Sánchez, G., Prado-Barragán, L. A., Rodríguez-Herrera, R., Martínez-Hernandez, J. L., & Contreras-Esquivel, J. C. (2008). Am J Bioch Biotech, 4, 354–366.
Fergucs, L. (1969). Mycol, 61, 120–129.
Tanseym, R. (1971). Arcltiv Fiir Mikrobiol, 77, 1–1I.
Somkuti, G. A. (1974). J Gener Microbiol, 81, 1–6.
Villena, G. K., & Gutiérrez-Correa, M. (2003). Rev Perú Biol, 10, 78–87.
Balasingham, J., Warburton, D., Dunhill, P., & Lilly, M. (1972). Biochim Biophys Acta, 7, 276–250.
Axelsson, A., & Persson, B. (1987). Appl Biochem Biotechnol, 16, 231–250.
Réczey, K., Stalbrand, H., Hahn-Hägerdal, B., & Tjerneld, F. (1992). Appl Microbiol Biotechnol, 38, 393–397.
Nagy, V., Toke, E. R., Keong, L. C., Szatzker, G., Ibrahim, D., Omar, I. C., et al. (2006). J Mol Catal B Enzym, 39, 141–148.
Fernandes, M. L. M., Saad, E. B., Meira, J. A., Ramos, L. P., Mitchell, D. A., & Krieger, N. (2007). Mol Catal B Enzym, 44, 8–13.
Domínguez-Malfavón, L. (2003). MS thesis, Universidad Autónoma de Coahuila, Saltillo, Coahuila, Mexico.
Savidge, T. A., & Cole, M. (1975). In J. H. Hash (Ed.), Methods in enzymology: penicillin acylase (bacterial) (Vol. 43, pp. 705–721). London: Academic.
Acknowledgement
The authors are grateful to the Universidad Autónoma de Coahuila (Mexico) for its financial support for this work. M. Mata-Gómez thanks CONACYT (Mexican Council of Science and Technology) for his fellowship to conduct this study. The authors are also grateful for the valuable help of Siong N.G. for reviewing this paper for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martínez-Hernández, J.L., Mata-Gómez, M.A., Aguilar-González, C.N. et al. A Process to Produce Penicillin G Acylase by Surface-Adhesion Fermentation Using Mucor griseocyanus to Obtain 6-Aminopenicillanic Acid by Penicillin G Hydrolysis. Appl Biochem Biotechnol 160, 2045–2053 (2010). https://doi.org/10.1007/s12010-009-8768-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8768-8