Abstract
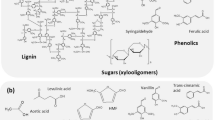
The recalcitrance of lignocellulosic biomass to enzymatic release of sugars (saccharification) currently limits its use as feedstock for biofuels. Enzymatic hydrolysis of untreated aspen wood releases only 21.8% of the available sugars due primarily to the lignin barrier. Nature uses oxidative enzymes to selectively degrade lignin in lignocellulosic biomass, but thus far, natural enzymes have been too slow for industrial use. In this study, oxidative pretreatment with commercial peracetic acid (470 mM) removed 40% of the lignin (from 19.9 to 12.0 wt.% lignin) from aspen and enhanced the sugar yields in subsequent enzymatic hydrolysis to about 90%. Increasing the amount of lignin removed correlated with increasing yields of sugar release. Unfortunately, peracetic acid is expensive, and concentrated forms can be hazardous. To reduce costs and hazards associated with using commercial peracetic acid, we used a hydrolase to catalyze the perhydrolysis of ethyl acetate generating 60–70 mM peracetic acid in situ as a pretreatment to remove lignin from aspen wood. A single pretreatment was insufficient, but multiple cycles (up to eight) removed up to 61.7% of the lignin enabling release of >90% of the sugars during saccharification. This value corresponds to a predicted 581 g of fermentable sugars from 1 kg of aspen wood. Improvements in the enzyme stability are needed before the enzymatically generated peracetic acid is a commercially viable alternative.






Similar content being viewed by others
References
Mooney, C. A., Mansfield, S. D., Touhy, M. G., & Saddler, J. N. (1998). Bioresource Technology, 64, 113–119.
Chernoglazov, V. M., Ermolova, O. V., & Klyosov, A. A. (1988). Enzyme and Microbial Technology, 10, 503–507.
Converse, A. O., Ooshima, H., & Burns, D. S. (1990). Applied Biochemistry and Biotechnology, 24–25, 67–73.
Sewalt, V. J. H., Glasser, W. J., & Beauchemin, K. A. (1997). Journal of Agricultural and Food Chemistry, 45, 1823–1828.
Pan, X., Xie, D., Gilkes, N., Gregg, D. J., & Saddler, J. N. (2005). Applied Biochemistry and Biotechnology, 121–124, 1069–1079.
Berlin, A., Balakshin, M., Gilkes, N., Kadla, J., Maximenko, V., Kubo, S., et al. (2006). Journal of Biotechnology, 125, 198–209.
Palonen, H., Tjerneld, F., Zacchi, G., & Tenkanen, M. (2004). Journal of Biotechnology, 107, 65–72.
Shimada, M., Ohta, A., Kurosaka, H., Hattori, T., Higuchi, T., & Takahashi, M. (1989). Roles of secondary metabolism of wood rotting fungi in biodegradation of lignocellulosic materials. ACS Symposium Series 399 (Plant Cell Wall Polym.), pp. 412–425.
Chung, N., Lee, I.-S., Song, H.-S., & Bang, W.-G. (2000). Journal of Microbiology and Biotechnology, 10, 737–752.
Hossain, S. M., Anantharaman, N., & Das, M. (2007). Journal of the Chemical Engineering Division of the Institution of Engineers (India), 87, 42–50.
Bergius, F. (1937). Industrial and Engineering Chemistry, 29, 247–253.
Funazukuri, T., Hirota, T., Nagatake, T., & Goto, M. (2000). The effects of additives on hydrolysis of cellulose with water under pressure. Endo, I., Nagamune, T., Katoah, S., & Yonemoto, T. eds., Bioseparation Engineering, Elsevier, New York, 16, pp. 181–185.
Nathan, S. N., Christin, M. L., & Michael, R. L. (2002). Biotechnology and Bioengineering, 79, 610–618.
Poljak, A. (1948). Angewandte Chemie, 60, 45–46.
Pan, G. X., Spencer, L., & Leary, G. J. (1999). Journal of Agricultural and Food Chemistry, 47, 3325–3331.
Sakai, K., Kuroda, Ken-ichi, & Kishimoto, S. (1972). Tappi Journal, 55, 1702–1706.
Sarkanen, K. V., & Suzuki, J. (1965). Tappi Journal, 48, 459–464.
Farrand, J. C., & Johnson, D. C. (1971). Journal of Organic Chemistry, 36, 3606–3612.
Nimz, H. H., & Schwind, H. (1979). Cellulose Chemistry and Technology, 13, 35–46.
Lawrence, W., McKelvey, R. D., & Johnson, D. C. (1980). Svensk Papperstidning, 83, 11–18.
Lai, Y.-Z., & Sarkanen, K. V. (1968). Tappi Journal, 51, 449–453.
Palonen, H., Thomsen, A. B., Tenkanen, M., Schmidt, A. S., & Viikari, L. (2004). Applied Biochemistry and Biotechnology, 117, 1–17.
Gharpuray, M. M., Lee, Y.-H., & Fan, L. T. (1983). Biotechnology and Bioengineering, 25, 157–172.
Kamstra, L. D., Ronning, D., Walker, H. G., Kohler, G. O., & Wayman, O. (1980). Journal of Animal Science, 50, 153–159.
Zhao, X., Wang, L., & Liu, D. (2007). Journal of Chemical Technology & Biotechnology, 82, 1115–1121.
Zhao, X., Wang, L., & Liu, D. (2008). Journal of Chemical Technology & Biotechnology, 83, 950–956.
Zhao, X., Zhang, L., & Liu, D. (2008). Bioresource Technology, 99, 3729–3736.
Ando, S., Kakimoto, T., Itoh, K., Arai, I., Kiyoto, K., & Hanai, S. (1988). Biotechnology and Bioengineering, 31, 802–804.
Zhaoxin, L., & Kumakura, M. (1995). Isotopes in Environmental and Health Studies, 31, 151–160.
Teixeira, L. C., Linden, J. C., & Schroeder, H. A. (1999). Applied Biochemistry and Biotechnology, 77–79, 19–34.
Teixeira, L. C., Linden, J. C., & Schroeder, H. A. (2000). Applied Biochemistry and Biotechnology, 84–86, 111–127.
Based on a cost of US$18 per liter for 35 wt% peracetic acid (100-L drum, Spectrum Chemicals) and a theoretical maximum conversion of 346 liters of ethanol per dry metric ton of hardwood biomass (101 gallons per dry US ton) (DOE Biomass Program. 2006. Theoretical Ethanol Yield Calculator. http://www1.eere.energy.gov/biomass/ethanol_yield_calculator.html).
Björkling, F., Frykman, H., Godtfredsen, S. E., & Kirk, O. (1992). Tetrahedron, 48, 4587–4592.
Picard, M., Gross, J., Lubbert, E., Tölzer, S., Krauss, S., van Pée, K.-H., et al. (1997). Angewandte Chemie International Edition in English, 36, 1196–1199.
Bernhardt, P., Hult, K., & Kazlauskas, R. J. (2005). Angewandte Chemie. International Edition, 44, 2742–2746.
Zhao, X., Zhang, T., Zhou, Y., & Liu, D. (2007). Journal of Molecular Catalysis A: Chemical, 271, 246–252.
Zhao, X., Cheng, K., Hao, J., & Liu, D. (2008). Journal of Molecular Catalysis A: Chemical, 284, 58–68.
Jencks, W. P., & Gilchrist, M. (1964). Journal of the American Chemical Society, 86, 4651–4654.
Ankudey, E. G., Olivo, H. F., & Peeples, T. L. (2006). Green Chemistry, 8, 923–926.
Mathews, I., Soltis, M., Saldajeno, M., Ganshaw, G., Sala, R., Weyler, W., et al. (2007). Biochemistry, 46, 8969–8979.
DiCosimo, R., Payne, M. S., Croud, V. B., Gavagan, J. E., Wagner, L. W., & Hann, E. C. (2007). Enzymatic Production of Peracids Using Perhydrolytic Enzymes. U. S. Patent Application No. 2007/0042924 A1, E. I. Du Pont DE Nemours and Company.
Mansfield, S. D., & Weineisen, H. (2007). Journal of Wood Chemistry and Technology, 27, 135–151.
Kong, F., Engler, C. R., & Soltes, E. J. (1992). Applied Biochemistry and Biotechnology, 34–35, 23–35.
Greenspan, F. P., & MacKellar, D. G. (1948). Analytical Chemistry, 20, 1061–1063; see also http://www.fmcchemicals.com/LinkClick.aspx?fileticket=9fuL5vDTWwM%3d&tabid=1466 &mid = 2560.
Pinkernell, U., Effkemann, S., & Karst, U. (1997). Analytical Chemistry, 69, 3623–3627.
Adney, B., & Baker, J. (1996). Measurement of Cellulase Activities. LAP-006 NREL Analytical Procedure, National Renewable Energy Laboratory, Golden, CO. From http://www.nrel.gov/biomass/pdfs/42628.pdf.
Wood, T. M., & Bhat, K. M. (1988). Methods for measuring cellulase activities, vol. 160. In W. A. Wood & S. T. Kellog (Eds.), Methods in enzymology (pp. 87–112). New York: Academic Press Inc.
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., & Crocker, D. (2006). Determination of Total Solids in Biomass. NREL Laboratory Analytical Procedure, National Renewable Energy Laboratory, Golden, CO. From http://devafdc.nrel.gov/pdfs/9572.pdf.
Brown, L., & Torget, R. (1995). Enzymatic saccharification of lignocellulosic biomass. NREL Laboratory Analytical Procedure LAP #009, National Renewable Energy Laboratory, Golden, CO. From http://www1.eere.energy.gov/biomass/analytical_procedures.html#LAP– 009.
Zhang, B., von Keitz, M., & Valentas, K. (2008). Applied Biochemistry and Biotechnology, 147, 143–150.
Although many other researchers pretreated with peracetic acid at 60°C (Ref. [52]), we found that peracetic acid degraded quickly to hydrogen peroxide at this temperature. After 5 h of pretreatment of aspen with 470 mM peracetic acid at room temperature, 86% of the peracetic acid remained, while at 60°C only 8% of remained. During this time 4% of the peracetic acid had decomposed to hydrogen peroxide at room temperature, but 33% had decomposed to hydrogen peroxide at 60°C.
Sun, R. C., Tomkinson, J., Zhu, W., & Wang, S. Q. (2000). Journal of Agricultural and Food Chemistry, 48, 1253–1262.
Grohmann, K., Mitchell, D. J., Himmel, M. E., Dale, B. E., & Schroeder, H. A. (1989). Applied Biochemistry and Biotechnology, 20–21, 45–61.
Kong et al (Ref. [43]) used the 2,4-dinitrosalicyclic acid colorimetric method to measure reducing sugar levels, which includes oligomers with with newly exposed reducing ends in addition to free monomeric sugars. For discussion, see Ref. [55].
Rivers, D. B., Gracheck, S. J., Woodford, L. C., & Emert, G. H. (1984). Biotechnology and Bioengineering, 26, 800–802.
Chang, V. S., & Holtzapple, M. T. (2000). Applied Biochemistry and Biotechnology, 84–86, 5–37.
Carboni-Oerlemans, C., de María, P. D., Tuin, B., Bargeman, G., van der Meer, A., & van Gemert, R. (2006). Journal of Biotechnology, 126, 140–151.
Chen, F., & Dixon, R. A. (2007). Nature Biotechnology, 25, 759–761.
Lu, Y., Yang, B., Gregg, D., Saddler, J. N., & Mansfield, S. D. (2002). Applied Biochemistry and Biotechnology, 98–100, 641–654.
McGinnis, G. D., Wilson, W. W., & Mullen, C. E. (1983). Industrial and Engineering Chemistry Product Research and Development, 22, 352–357.
Palonen, H., & Viikari, L. (2004). Biotechnology and Bioengineering, 86, 550–557.
Pan, X. J., Gilkes, N., & Saddler, J. N. (2006). Holzforschung, 60, 398–401.
Cherry, J. R., Lamsa, M. H., Schneider, P., Vind, J., Svendsen, A., Jones, A., et al. (1999). Nature Biotechnology, 17, 379–384.
Acknowledgements
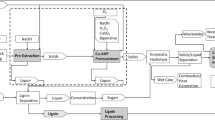
We thank the University of Minnesota’s Institute on the Environment for financial support. We thank Bruce Dale (U. Michigan) for providing a template for the mass balance figures and Jacob Tewalt and Dahai Yu for preliminary experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duncan, S., Jing, Q., Katona, A. et al. Increased Saccharification Yields from Aspen Biomass Upon Treatment with Enzymatically Generated Peracetic Acid. Appl Biochem Biotechnol 160, 1637–1652 (2010). https://doi.org/10.1007/s12010-009-8639-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8639-3