Abstract
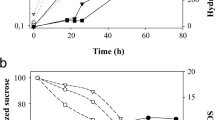
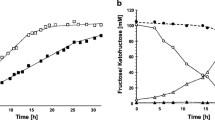
The enzyme β-d-fructofuranosidase fructohydrolase (FFH) cleaves the α-1,4 glycosidic linkage between α-d-glucose and β-d-fructose molecules of sucrose, releasing monosaccharides by hydrolysis. In the present study, FFH production in Candida utilis GC-46, a lipolytic wild yeast strain was improved by exposure to N-methyl N-nitro N-nitroso guanidine (NG) and 2-deoxy-d-glucose (2dg) at various levels. The mutant strain NG-5 was obtained after exposure to 0.06 mg/ml of NG for 20 min. NG-5 offers improved extracellular FFH production (34 ± 2.6 U/ml/min) when compared to the wild strain (1.15 ± 0.01 U/ml/min). A 40-fold increase of FFH (45.65 ± 2.0 U/ml/min) was achieved when the process parameters, including incubation period (48 h), sucrose concentration (5.0 g/l), initial pH (6.0), inoculum size (2.0% v/v, 16 h old), and urea concentration (0.2%, w/v) were identified using Plackett–Burman design. The kinetic parameters viz. Q p (0.723 U/g/h), Y p/s (2.036 U/g), and q p (0.091 U/g yeast cells/h) indicate that NG-5 is a hyperproducer of extracellular FFH with a concomitant increase in growth rate. The volumetric productivity of NG-5 was over sixfold improved over the parental strain. The enzyme production improvement is highly significant (HS, LSD 0.042, p ≤ 0.05), indicating commercial utility.





Similar content being viewed by others
References
Barlikova, A., Svorc, J., & Miertus, S. (1991). Analytical Chemistry, 247, 83–87.
Dynesen, J., Smits, H. P., Olsson, L., & Nielsen, J. (1998). Applied Microbiology and Biotechnology, 50, 579–582. doi:10.1007/s002530051338.
Rincon, A. M., Codon, A. C., & Benitez, T. (2001). Applied and Environmental Microbiology, 67, 4279–4285. doi:10.1128/AEM.67.9.4279-4285.2001.
Sanchez, M. P., Huidobro, J. F., Mato, I., & Sancho, M. T. (2001). Journal of Agricultural and Food Chemistry, 49, 416–422. doi:10.1021/jf0003350.
Roitsch, T., Balibrea, M. E., Hofmann, M., & Sinha, A. K. (2003). Journal of Experimental Botany, 54, 513–524. doi:10.1093/jxb/erg050.
Neto, J., Infanti, P., & Vitolo, M. (1996). Applied Biochemistry and Biotechnology, 58, 407–412. doi:10.1007/BF02941720.
Murray, R., Walsh, K., & Foley, G. (2007). Biotechnology Progress, 23, 79–87.
Haq, I., Ali, S., & Baig, M. A. (2004). Biologia, 50, 25–33.
Ginka, I. F., Emilina, D. S., & Dora, M. B. (2004). Zurich Naturforsch, 59, 99–103.
Miller, G. L. (1959). Analytical Chemistry, 31, 426–428. doi:10.1021/ac60147a030.
Akgol, S., Kacarb, Y., Denizlia, A., & Aricab, M. Y. (2001). Food Chemistry, 74, 281–288. doi:10.1016/S0308-8146(01) 00150-9.
Pirt, S. J. (1975). In principles of microbe and cell cultivation (2nd ed.), pp. 116–124. London: Blackwell Scientific.
Snedecor, G., & Cochran, W. G. (1980). In statistical methods (7th ed.), pp. 80–86. Iowa: Iowa State University.
Plackett, R. L., & Burman, J. P. (1946). Biometrika, 33, 305–325. doi:10.1093/biomet/33.4.305.
Ahuja, S. K., Ferreira, G. M., & Morreira, A. R. (2004). Biotechnology and Bioengineering, 85, 666–675. doi:10.1002/bit.10880.
Herwig, C., Doerries, C., Marison, I., & Stockar, U. V. (2001). Biotechnology and Bioengineering, 82, 247–258. doi:10.1002/bit.10004.
Haq, I., Baig, M. A., & Ali, S. (2005). World Journal of Microbiology & Biotechnology, 21, 287–292.
Bokosa, I., Krastanov, A., & Roshkova, Z. I. (1992). Nauchni Tritical, 39, 269–279.
Vitolo, M., Duranti, M. A., & Pellegrim, M. B. (1995). Journal of Industrial Microbiology, 15, 75–79. doi:10.1007/BF01569803.
Persike, D. S., Bonfim, T. M., Santos, M. H., Lyng, S. M., Chiarello, M. D., & Fontana, J. D. (2002). Bioresource Technology, 82, 79–85. doi:10.1016/S0960-8524(01) 00121-3.
Gancedo, J. M. (1998). Microbiology and Molecular Biology Reviews, 62, 334–361.
Hashimoto, S., Ogura, M., Aritomo, K., Hoshida, H., Nishizawa, Y., & Akada, R. (2005). Applied and Environmental Microbiology, 71, 312–319. doi:10.1128/AEM.71.1.312-319.2005.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, S., Ashiq, M. Enhanced Production of an Extracellular β-d-Fructofuranosidase Fructohydrolase from a 2-Deoxy-d-glucose Stabilized Mutant of Candida utilis . Appl Biochem Biotechnol 159, 453–463 (2009). https://doi.org/10.1007/s12010-009-8575-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8575-2