Abstract
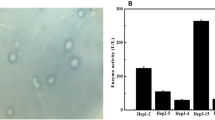
A heparinase-producing fungus was isolated, and the strain was taxonomically characterized as Aspergillus flavus by morphophysiological and 26S rRNA gene homology studies. The culture produced intracellular heparinase enzyme, which was purified 40.5-fold by DEAE-Sephadex A-50, CM-Sephadex C-50, and Sephadex G-100 column chromatography. Specific activity of the purified enzyme was found to be 44.6 IU/μg protein and the molecular weight of native as well as reduced heparinase was 24 kDa, showing a monomeric unit structure. Peptide mass spectrum showed poor homogeneity with the database in the peptide bank. The enzyme activity was maximum at 30 °C in the presence of 300 mM NaCl at pH 7.0. In the presence of Co2+, Mn2+ ions, and reducing agents (β-mercaptoethanol, dithiothreitol), enzyme activity was enhanced and inhibited by iodoacetic acid. These observations suggested that free sulfohydryl groups of cysteine residues were necessary for catalytic activity of the enzyme. The enzyme was also inhibited by histidine modifier, DEPC, which suggests that along with cysteine, histidine may be present at its active site. The enzyme showed a high affinity for heparin as a substrate with K m and V max as 2.2 × 10−5 M and 30.8 mM min−1, respectively. The affinity of the enzyme for different glycosaminoglycans studied varied, with high substrate specificity toward heparin and heparin-derived polysaccharides. Depolymerization of heparin and fractionation of the oligosaccharides yielded heparin disaccharides as main product.






Similar content being viewed by others
References
Linhardt, R. J., & Gunay, N. S. (1999). Seminars in Thrombosis and Hemostasis, 25(Suppl 3), 5–16. doi:10.1055/s-2007-996417.
Segal, J. B., Eng, J., Jenckes, M. W., Tamariz, L. J., Bolger, D. T., Krishnan, J. A., et al. (2003). Evidence Report/Technology Assessment (Summary)1–6.
Yoshida, E., Sakai, K., Tokuyama, S., Miyazono, H., Maruyama, H., Morikawa, K., et al. (2002). Bioscience, Biotechnology, and Biochemistry, 66, 1181–1184. doi:10.1271/bbb.66.1181.
Kim, W. S., Kim, B. T., Kim, D. H., & Kim, Y. S. (2004). Journal of Biochemistry and Molecular Biology, 37, 684–690.
Yapeng, C., Ningguo, G., Xiulan, C., Jing, Y., Shijun, Q., & Shuzheng, Z. (2003). Journal of Biochemistry, 134, 365–371. doi:10.1093/jb/mvg154.
Zimmerman, J., Lewis, N., & Heft, R.(1993) Method for the enzymatic neutralization of heparin, US Patent 5,262,325.
van den Besselaar, A. M., & Meeuwisse-Braun, J. (1993). Blood Coagulation & Fibrinolysis, 4, 635–638. doi:10.1097/00001721-199308000-00016.
Dongfang, L., Pojasek, K., Shriver, Z., Holley, K., El-shabrawi, Y., Venkataraman, G., et al. (2002) Heparinase III and uses thereof, EP Patent 1,266,013.
Sasisekharan, R., Moses, M. A., Nugent, M. A., Cooney, C. L., & Langer, R. (1994). Proceedings of the National Academy of Sciences of the United States of America, 91, 1524–1528. doi:10.1073/pnas.91.4.1524.
Shaya, D., Tocilj, A., Li, Y., Myette, J., Venkataraman, G., Sasisekharan, R., et al. (2006). The Journal of Biological Chemistry, 281, 15525–15535. doi:10.1074/jbc.M512055200.
Joubert, J., & Pitout, M. (1985). Cellular and Molecular Life Sciences, 41, 1541.
Zimmermann, J., Su, H., Blain, F., Bennett, C., Gu, K., & Musil, R.(2003) Nucleic acid sequences and expression systems for heparinase III derived from flavobacterium heparinum, EP Patent 0,763,101.
Varga, J., Juhász, Á., Kevei, F., & Kozakiewicz, Z. (2004). European Journal of Plant Pathology, 110, 627–640. doi:10.1023/B:EJPP.0000032402.36050.df.
Banga, J., Tripathi, C. K. M., & Bihari, V. (2008). Medicinal Chemistry Research, 17, 85–93. doi:10.1007/s00044-007-9039-2.
Linker, A., & Hovingh, P. (1972). In U. Ginsburg (Ed.), Methods in enzymology, vol. 28: Heparinase and heparitinase from flavo-bacteria. (pp 902–911). New York: Academic.
Lohse, D., & Linhardt, R. (1992). The Journal of Biological Chemistry, 267, 24347–24355.
Rosenfeld, J., Capdevielle, J., Guillemot, J. C., & Ferrara, P. (1992). Analytical Biochemistry, 203, 173–179. doi:10.1016/0003-2697(92)90061-B.
Gao, J., Liu, Z., & Yu, J. (2007). Mycopathologia, 164, 91–95. doi:10.1007/s11046-007-9029-4.
Nikkuni, S., Nakajima, H., Hoshina, S. I., Ohno, M., Suzuki, C., Kashiwagi, Y., et al. (1998). The Journal of General and Applied Microbiology, 44, 225–230. doi:10.2323/jgam.44.225.
Sasisekharan, R., Lohse, D., Cooney, C., Linhardt, R., & Langer, R.(1996) Purification, composition and specificity of heparinase I, II, and III from flavobacterium heparinum, US Patent 5, 569, 600.
Bohmer, L. H., Pitout, M. J., Steyn, P. L., & Visser, L. (1990). The Journal of Biological Chemistry, 265, 13609–13617.
Ernst, S., Langer, R., Cooney, C. L., & Sasisekharan, R. (1995). Critical Reviews in Biochemistry and Molecular Biology, 30, 387–444. doi:10.3109/10409239509083490.
Godavarti, R., & Sasisekharan, R. (1998). The Journal of Biological Chemistry, 273, 248–255. doi:10.1074/jbc.273.1.248.
Shriver, Z., Hu, Y., Pojasek, K., & Sasisekharan, R. (1998). The Journal of Biological Chemistry, 273, 22904–22912. doi:10.1074/jbc.273.36.22904.
Viskov, C., & Mourier, P. (2007) Oligosaccharides, preparation method and use thereof, and pharmaceutical compositions containing same, US patent 0, 142, 323.
Yamada, S., & Sugahara, K. (2003). In P. Thibault, & S. Honda (Eds.),Methods in molecular biology vol. 213: Preparation of oligosaccharides from sulfated glycosaminoglycans using bacterial enzymes (pp. 71–78). Clifton, NJ: Humana.
Acknowledgement
This research was financially supported by grant from University Grants Commision, South Campus, Delhi University, Benito Juarez Marg, New Delhi-110021.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banga, J., Tripathi, C.K.M. Purification and Characterization of a Novel Heparin Degrading Enzyme from Aspergillus flavus (MTCC-8654). Appl Biochem Biotechnol 160, 1004–1016 (2010). https://doi.org/10.1007/s12010-009-8530-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8530-2