Abstract
Due to technological advancement, environment suffers from untreated toxic heavy metal bearing effluent coming from different industries. Chromium (VI) is one of those heavy metals having adverse impact on ecological balance, human, and plant health because of its carcinogenic properties. Biosorption is presented as an alternative to traditional technologies which are costly and inefficient for treatment of industrial wastes containing low amount of heavy metals. In this study, bioremediation of Cr (VI) ions by immobilized Bacillus cereus M1 16 was investigated in a laboratory scale packed bed up-flow column reactor. The effect of important parameters, such as the inlet flow rate, influent concentration, and effective bed height, has been studied. External mass transfer, surface adsorption, and intrabead mass transfer were also studied to conclude the rate limiting step for removal of Cr (VI) and to determine the process parameters which are important for biosorption optimization. The external mass transfer coefficient was calculated at different flow rates (6.51 × 10−2 to 7.58 × 10−2 cm/min). Using the model, the surface adsorption rate constant (k ad) and the intrabead mass transfer coefficient (k i) were predicted as 0.0267 × 10−3 and 0.7465 × 10−3 l/g/min, respectively. Both are much lower than the external mass transfer coefficient (k e). The surface adsorption phenomenon is acting as the rate-limiting step due to its high resistance for removal of Cr (VI).
Similar content being viewed by others
Introduction
Environmental contamination is a major problem in recent years. As technology progress, the environment suffers from the detrimental effect of industrial effluents. Heavy metal removal from waste water is important for protection of the environment and human health. Heavy metals like mercury, cadmium, lead, nickel, and chromium are toxic, even in extremely minute quantities. Anthropogenic sources of chromium are industries, viz., electroplating, leather tanning, metal finishing, chemical industries, and many others. Chromium exists in several oxidation states out of which Cr (III) and Cr (VI) are most stable form. Because of its high toxicity and potential carcinogenicity, Cr (VI) is of special concern [1]. In humans, Cr (VI) causes severe diarrhea, ulcers, eye and skin irritation, kidney dysfunction, and probably lung carcinoma [2]. Tannery waste contains 80–250 mg/l Cr (VI), whereas the maximum contaminated level (MCL) for chromium in the domestic water supplies set by US Environmental Protection Agency and the European Union Directive to be 0.05 mg/l and recommended discharge value is less than 5 mg/l [3].
The metallic species are nonbiodegradable and therefore persist indefinitely, accumulating in living tissues throughout the food chain [4]. Conventional methods such as lime or caustic soda treatment, oxidation–reduction ion exchange, filtration, and evaporation recovery can be used for removing heavy metals from waste water but are expensive, insufficient for metal removal, and require costly equipment and high cost operation and energy. The search for alternative and innovative treatment in use of biological materials such as algae, fungi, yeast, and bacteria for the removal and recovery technologies has gained importance during recent years because of the better performance and low cost of these biological materials [5–7]. Commercial application of microbial biomass as a biosorbent, however, has been hindered by problems associated with physical characteristics of these materials such as small particle size with low density, poor mechanical strength and rigidity, and solid/liquid separation [8, 9]. Immobilization of biomass within a suitable matrix can overcome these problems by offering ideal size, mechanical strength, rigidity, and porous characteristics to the biological materials [10].
Although other type of reactors, e.g., batch or continuous stirrer tank reactors [11, 12], fluidized bed [13], and moving bed [14], are available, adsorption separation techniques are almost always carried out in packed bed column [15]. Fluidization of a bed requires a large input of power. Although the fluidized bed reactor works reasonably on a laboratory scale, it is difficult to predict the success of a scale-up procedure [16]. Despite the ease of fabrication and relative ease of environmental control, tank reactors are difficult to automate [16]. Packed bed adsorption has a number of advantages relative to its process engineering. It is a simple high yield operation and relatively easily scaled up from a laboratory scale procedure, the stages in the separation protocol can be automated, and high degree of purification can often be achieved in a single step process. For heavy metal biosorption, packed bed columns are generally used with immobilized cells [15]. A number of authors reported the use of continuous packed bed column reactor using immobilized biomass [12, 15, 17–19]. A novel type immobilized biomass biological reactor, in a moving bed sand filter configuration, has been evaluated in pilot scale for the removal of heavy metals in mining, metallurgical, and plating industrial effluents in the frame of a European Research Project [20]. The operation of the filter is based on the fact that sand grains act as support material for biomass immobilization. The microbial biofilm formed interacts with the soluble metal species of the wastewater treated.
Many mathematical models have also been used to study packed bed system, and dynamic behavior has been well described [21–24]. All these models have originated mainly from research on activated carbon sorption and ion exchange or chromatography application. However, in the case of Cr (VI) biosorption in a packed bed reactor, a few studies have been reported [25, 26] and there is no study on the rate-limiting step for Cr (VI) removal in the column reactor using immobilized biomass of Bacillus cereus.
The objective of this study is to investigate the removal of chromium from aqueous solution by immobilized B. cereus M1 16 in a packed bed up-flow column reactor. The effects of design parameters, such as flow rate, bed height, and inlet metal concentration, have been studied. In addition, kinetics of biosorption and rate-limiting step for removal of Cr (VI) from solution have been investigated.
Diffusion and Sorption Model
To develop the model, the ideas have been partially taken from the work of Aksu and Kutsal [15]. The assumptions of their model for Cu (II) biosorption using algal biomass in packed bed column reactor were (a) algal particles are nonporous, (b) spherical, and (c) only surface adsorption (SA) of Cu (II) metal ions occurs. But in the current study, the immobilized bacterial cells were used for Cr (VI) sorption. The calcium alginate gel beads containing biomass are generally porous [27, 28]. So, the assumptions used in this study were (a) isothermal process, (b) constant column void fraction, (c) the immobilized cell bead are porous, i.e., there are multiple channels inside the beads, (d) all immobilized beads are spherical, and (e) mainly three steps involved for biosorption of Cr (VI) metal ions: external diffusion from bulk solution to bead surface, surface adsorption in bead, and intra bead diffusion. Accordingly, the model has been extended. The differential mass balance in the packed bed column [29] is given in Eq. 1:
where C is the Cr (VI) concentration in liquid phase (mg/l), L is the axial coordinate with origin at the column inlet (cm), ɛ is the void fraction in packed bed and is the interstitial velocity of solution in the column (cm/min), ρ p is the immobilized bead density (mg/cm3), q is the metal concentration in the biosorbent averaged over the biosorbent volume (mg/g), D L is the axial dispersion coefficient, and t represents the time (min). Neglecting axial dispersion, considering a steady state (equilibrium) before the break point time (the time when the outlet effluent concentration start to increase sharply), Eq. 1 can be written as,
Considering \(G = \rho _{\text{p}} \left( {1 - \varepsilon } \right)A_{{\text{col}}} L\; \times \;10^{ - 3} \) and \(F = \varepsilon v_{{\text{sol}}} A_{{\text{col}}} \), Eq. 2 can be expressed as,
where F is the volumetric flow rate of metal ion into packed bed column (ml/min), G is the adsorbent amount in the packed bed (g), and A col is the cross-sectional area of the column (cm2).
The term \(\frac{{dq}}{{dt}}\) represents the local rate of Cr (VI) removal of the column by combined removal due to surface adsorption and intra bead diffusion (mg/g/min). Various dynamic models have been derived which differs mainly in the choice of kinetic rate expression. It is assumed that the net removals of Cr (VI) by SA and intrabead diffusion (IBD) are saturation type phenomena. So, the relation between removal rate of Cr (VI) by SA plus IBD and the metal ion concentration at equilibrium in the column is given by,
where k aid and K are the removal rate constant (l/g/min) by SA plus IBD and removal constant (l/g), respectively. As Eq. 3 is used in Eq. 4, it becomes,
Eq. 6 formed by integrating Eq. 5 using the boundary condition at L = 0; C = C0 and at L = L; C = C:
where C 0 and C are the inlet metal ion concentration (mg/l) and outlet metal ion concentration (mg/l), respectively. k aid and K can be determined from \({\text{1n}}\frac{{C_{\text{0}} }}{C}\;{\text{vs}}{\text{.}}\;\left( {C_{\text{0}} - C} \right){\text{plot}}\).
Now, the average metal ion transport from bulk solution to immobilized bead surface in the packed bed column can be correlated with some important dimensionless numbers which characterize the flow conditions. The external mass transfer (bulk to bead surface) coefficient can be estimated using Wakao and Funazkri correlation (Eq. 7) [30] by relating Sherwood number (N sh), Reynolds number (N Re ), and Schmidt number (N sc)
The Sherwood number is related to external mass transfer coefficient and external diffusivity by,
where k e is the external mass transfer coefficient between the immobilized beads and metal ions solution (cm/min), d m is the diameter of immobilized bead (cm), and D e is the external diffusivity of metal ion solution (cm2/min).
The theory of dilute diffusion of electrolyte is well developed and for dilute solution of a single electrolyte, the well-known Nernst–Haskell equation (Eq. 9) is applicable to define the external diffusivity of the Cr (VI) ions bulk solution [31].
where R is the universal gas constant (J/mol/K), T is the absolute temperature (K), F N is the Faraday’s constant (96,500 coulomb/gequive), z + and z − are the valences of cation and anion, respectively, and λ + and λ − are the cationic and anionic conductance at infinite dilution (mhos/gequive). The multiplication factor 60 comes in Eq. 9 due to transformation of unit of D e from cm2/s to cm2/min.
Now, the Reynolds number in packed bed column reactor is defined as follows:
where ρ sol is the density of metal containing solution (g/ml) and μ sol is the viscosity of metal containing solution (g/cm/min).
The Schmidt number in packed bed is defined as,
The value of k e can be determined from Eq. 7 at different flow rates of Cr (VI) metal ion solution in packed bed reactor. The intrabead diffusivity due to porosity in the bead can be estimated using Urano and Tachikawa equation [32] as,
where D i is the intrabead diffusivity (cm2/min), t is the time (min), and q and q e are the metal uptake at any time (mg/g) and metal uptake at equilibrium (mg/g), respectively.
Now, it was assumed that there is a negligible effect of flow rate on intrabead diffusivity. Therefore, to estimate q and q e, the batch experiments data (shake flask experiments data) can be used. These are as follows:
where C 0b is the initial Cr (VI) concentration in shake flask (mg/l), C b is the Cr (VI) concentration at time t in shake flask (mg/l), C eb is the equilibrium Cr (VI) concentration in shake flask (mg/l), V is the volume of Cr (VI) solutions in shake flask (ml), and G b is the amount of biosorbent in shake flask.
So, Eq. 15 can be derived using Eqs. 13 and 14 in Eq. 12.
Now, the solid phase (intrabead) mass transfer coefficient (k i) can be estimated using the following relation [33]:
where ϕ s is the sphericity of the immobilized bead.
Considering the external mass transfer rate, adsorption rate, and intrabead mass transfer rate together, Cr (VI) metal ions are transported from the bulk liquid to the liquid–solid interface (external surface of immobilized bead) and adsorption occurred at the outer surface of the immobilized bead and also metal ions are diffused from the surface of the bead to the inner surface of the bead. The transport rate of Cr (VI) ion to the surface of immobilized bead can be expressed as
where r s is the external mass transfer rate (mg/g/min), A p is the external surface area of per unit weight of immobilized bead (cm2/g), and C S is the metal ion concentration adsorbed onto the immobilized bead (mg/l).
Now, the removal of Cr (VI) by combination of surface adsorption and intrabead diffusion rate step may be represented by a single first-order removal rate equation, specially at low metal ion concentration. In this study, it was also proved that this is a first-order removal process. So, the first-order removal rate by SA and IBD can be represented as,
Now, the total resistance, \(\left( {\frac{1}{{k_{{\text{aid}}} }}} \right)\) due to the SA and IBD can be divided into two parts: resistance due to SA \(\left( {\frac{1}{{\,k_{{\text{ad}}\,} }}} \right)\) and resistance by IBD \(\left( {\frac{1}{{\,k_{\text{i}} }}} \right)\); it can be written as,
where k ad is the SA rate constant (l/g/min).
At equilibrium, r s = r aid, i.e.,
The total removal rate by SA and IBD can be expressed using Eq. 18 in terms of C in Eq. 22 as,
and k o is the overall first-order removal rate constant (l/g/min).
The total amounts of adsorbed Cr (VI) ion in column experiments (M u, column uptake, mg) for a given feed concentration and flow rate were calculated from the area under the curve C ad (= C 0 − C) vs. time (t). It can be expressed as,
The specific Cr (VI) uptake is defined as the total amount of metal ion adsorbed per gram of adsorbent in the packed bed at the end of total flow time. Amount of total metal ion entered to the column (M total) can be calculated from the Eq. 25 as,
The total removal percentage (%RM) is also calculated in Eq. 26,
Experimentally, Cr (VI) removal rate (r aide) can be calculated in Eq. 27,
The residence time (τ) of solution in the column can be calculated using Eq. 28,
Materials and Methods
Microorganism
A mutated strain of B. cereus M1 16 [34] was used in the present study. The strain was maintained on nutrient agar by monthly subculturing and stored at 4 °C.
Biomass Cultivation and Immobilization
Inoculum was prepared by transferring one loopfull of cells from slant culture of B. cereus M1 16 to 50 ml sterile nutrient broth having the composition (g/l) beef extract 1.0, yeast extract 2.0, peptone 5.0, and NaCl 5.0 (pH 6.5) in 250 ml Erlenmeyer flask and incubating at 30 ±1 °C and 120 rpm for 24 h. Fifty milliliters of fermentation medium (composition: same as inoculum medium) in 250 ml Erlenmeyer flask was inoculated with 4% (v/v) inoculum and incubated at 30 °C for 24 h at 120 rpm. Biomass was harvested by centrifugation at 5,500 rpm for 15 min. After washing twice with normal saline, the required amount of wet biomass was used for immobilization. Immobilization was carried out with wet biomass of B. cereus M1 16. One milliliter of cell suspension in normal saline was mixed thoroughly with 2 ml sodium alginate (final concentration 3%) and was added to 2% calcium chloride solution drop by drop using a hypodermic syringe and allowed to stand for 2 h at 4 °C. Then the beads were washed thoroughly with distilled water followed by 0.2 M citrate buffer (pH 3.0) and air dried. For storage, beads were dipped in 0.2 M citrate buffer (pH 3.0) and stored at 4 °C until further use. The average diameter of the resultant beads was 0.38 cm.
Synthetic Metal Solution
The standard stock solution (500 mg/l) of chromium was prepared by dissolving appropriate amount of potassium dichromate (K2Cr2O7) in distilled water. The working metal solutions (10, 20, and 50 mg/l) were prepared by dilution of stock solution using citrate buffer solution of appropriate pH.
Experimental Design
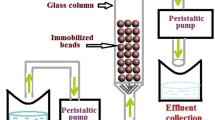
The experimental setup of the packed bed column rector is schematically shown in Fig. 1. Experiments were carried out in a glass column (65 cm length and internal diameter 1.094 cm) varying the effective bed height 60, 45, and 35 cm using immobilized biomass. The solution of potassium dichromate with different initial concentration (C o) was introduced continuously from the bottom of the column over the immobilized bead at different flow rate using a variable speed peristaltic pump. Glass wool was placed at the bottom of the column to ensure homogeneous distribution of the feeding solution and to protect the immobilized beads. The residual concentration of Cr (VI) (C) in effluent was measured at regular time intervals.
Estimation of Chromium
Residual concentration of chromium (VI) in the column effluent fluid (for column study) and culture broth (for shake flask experiments) was estimated using an absorption spectrophotometer (Varian 1656)
Dry Biomass
Washed biomass (wet) from a measured amount of whole fermentation broth was taken in a previously weighed aluminum cup and dried at 70 °C overnight and was weighed again. The weight of the dry cell mass was calculated by finding the difference between those two.
Determination of Column Void Fraction
After introducing the immobilized bead into the column, the effective column was filled by water and then the porous beads were allowed to be filled by the water and after some time, the remaining water of the column was removed. Then again, the effective column was filled by the water and again the water was removed from the column and the volume of the water (W) was measured. Then void fraction (ɛ) can be calculated using this volume and the total volume of the effective column (W h).The expression is
and
where d col is the internal diameter of the column.
Experiments for Determination of Intrabead Diffusivity
A set of well-organized batch experiments were conducted in shake flask to determine the intrabead diffusivity (D i), assuming very less effects of flow rate of metal containing solution in column on D i. Different initial concentrations of metal ion (range 25–300 mg/l) were used with the immobilized beads having same biomass concentration (as in column reactor) at pH 3.0, temperature 30 °C, and 120 rpm in shake flasks with working volume of 50 ml in 250 ml Erlenmeyer flask. Cr (VI) concentrations in the sample collected at different time intervals were estimated till equilibrium was reached.
Optimum pH (3.0), temperature (25 °C), and biomass concentration (0.0326 g dry biomass/g of alginate bead) for biosorption of Cr (VI) using the mutated B. cereus M1 16 were reported earlier [35]. Those parameters were kept constant throughout the experiments.
Results and Discussion
Effect of Flow Rate and Column Bed Height
In the first stage of the column study, the flow rate of aqueous solution containing 20 mg/l Cr (VI) in 0.2 M citrate buffer (pH 3.0) varied from 2.6 to 3.4 ml/min. The temperature was maintained at 25 °C and bed height of the column varied as 35, 45, and 60 cm. When the metal ion containing solution continued to flow, the bed became saturated with metal ions after a definite period and the concentration of solute in the effluent suddenly attained near about C o; the system had reached the breakpoint. The time required to reach the break point time decreases with increasing flow rate at a fixed bed height (Fig. 2). Percent removal also increases with decreasing in flow rate (Table 1). Much sharper breakthrough curves were obtained at higher flow rate. The main reason is that at higher flow rate, the residence time of the metal ion in the column was not long enough for the adsorption equilibrium to be attained; the metal ion solution might leave the column before the equilibrium occurred (Table 2). For that reason, adsorbed Cr (VI) concentration decreased with increasing flow rate. The removal amounts of Cr (VI) were calculated by multiplying the area under the curve C ad (= C 0 − C) vs. t up to equilibrium and corresponding flow rate (Eq. 24). As indicated in the Fig. 2, maximum sorption of Cr (VI) was observed at 60 cm bed height (q = 17.17 mg) with 55% removal (Table 1). As bed height increased, the total amount of biomass increased consequently the total number of active site and ionic group that adsorb Cr (VI) was increased and enhanced the removal percent (Table 1).
Effect of Initial Cr (VI) Concentration in Feed
To study the effect of initial Cr (VI) concentration (range 10–50 mg/l) in feed solution, the solution was passed over the packed bed (Fig. 1) with effective bed height 60 cm, flow rate 2.6 ml/min, pH 3.0, and temperature 25 °C. At lower inlet metal ion concentrations, breakthrough curves were dispersed in nature, breakthrough time was long, and the surface of the adsorbent was saturated with metal ion after a longer period of time (Fig. 3). At higher inlet metal ion concentration, breakthrough curves were much sharper (Fig. 3) and removal rate (using Eq. 27) was higher (Fig. 4). It was observed from that maximum Cr (VI) sorption (29.56 mg) occurred when inlet Cr (VI) concentration was 50 mg/l but percent removal reached maximum (64.09%) at 10 mg/l inlet Cr (VI) concentration (Table 1). The break point time decreased with increase in initial metal ion concentration as the binding sites became more quickly saturated in the system. A high concentration difference between solution and cell provides a high driving force for the removal process and thus higher removal capacity is achieved in the column fed with the influent having higher Cr (VI) concentration.
Column Kinetics Study
The dynamic of the removal of Cr (VI) for solution depends on external mass transfer from bulk solution to surface of the beads, surface adsorption, and intrabead diffusion. To study the effect of N Re on external mass transfer coefficient (k e), the flow rate varied from 2.6 to 3.4 ml min−1 keeping initial concentration 20 mg/l, pH 3, and temperature 25 °C. Reynolds number is dependent on flow rate and values were calculated using Eq. 10 with d m = 0.3775 cm, ρ sol = 0.9967 g/ml, ε = 0.275, A col = 93.99 × 10−2 cm2, and μ sol = 0.528 g/cm/min determined from experimental results. The values of Reynolds numbers varied from 7.17 to 9.38 as flow rate varied from 2.6 to 3.4 ml/min (Table 2). The external diffusivity (D e) of metal ion in solution at 25 °C (71.26 × 10−5cm2/min) was calculated using Eq. 9 considering the values of z + as 1, z − as 2, λ +, and λ − as 76 mhos/gequive and 48.613 mhos/gequive, respectively. Again, Schmidt number was determined (742.57) from Eq. 11 using the value of calculated D e. External mass transfer coefficients (k e) were determined applying Eqs. 7 and 8 and it plotted against Reynolds number in Fig. 5. It showed that N Re increased while residence time (τ) decreased and k e increased with increasing flow rate. The residence time (τ), Reynolds number (N Re ), external mass transfer coefficient (k e ), and equilibrium removal rate calculated from experiments (r aide) were shown in Table 2.
This indicates that external mass transfer resistance decreases with increase in flow rate. At higher flow rate, adsorption rate was low due to insufficient contact time for establishing equilibrium between bacterial cell mass and metal ions. As residence time of the solute in the column is not sufficient for equilibrium, the metal ion solution will leave the column before equilibrium occurs. The removal rate constant (k aid) and removal constant (K) can be calculated using the intercept and slope of \({\text{ln}}\frac{{C_{\text{0}} }}{C}\,\) vs. (C 0 − C) plot (Fig. 6) and the calculated values are 0.02579 × 10−3 l/g/min (Table 3) and 2.5 × 10−2 l/mg, respectively.
To determine the intrabead diffusivity, some batch experiments in shake flask were performed using immobilized beads of microorganism which has been described in “Materials and Methods” section. The dynamic profiles of Cr (VI) at different initial concentration in shake flask were shown in Fig. 7. The concentration of Cr (VI) at the end of each batch experiment was taken as equilibrium concentration (C eb) of that batch. Now, \( - {\text{ln}}\left[ {1 - \left( {\frac{{C_{{\text{0b}}} - \,\,C_{\text{b}} \,}}{{C_{{\text{0b}}} - \,\,C_{{\text{eb}}} }}} \right)^2 } \right]\,\) vs. t (time) was plotted for each experiment and it fitted well in a straight line with slope 0.0078 and good R 2 (0.91) value (Fig. 8). The intrabead diffusivity (D i) is calculated as 2.8181 × 10−5 cm2/min using Eq. 15 and slope of the straight line (0.0078; with d m = 0.3775 cm). Then, the intrabead mass transfer coefficient (k i) can be estimated using Eq. 16 with the help of calculated D i and ϕ s as 1 (sphericity, ϕ s is 1 because the immobilized beads were spherical).The calculated value k i is 0.7465 × 10−3 l/g/min (Table 3). Now, the surface adsorption rate constant (k ad) can be evaluated using Eq. 19 with the help of calculated values of k aid and k i and the value is 0.0267 × 10−3 l/g/min (Table 3). The overall first-order removal rate constant (k o) can be estimated using Eq. 23 with A p = 19.325 cm2/g. All the mass transfer coefficients and rate constants were listed in Table 3. The surface adsorption rate constant (k ad) is almost 50 times lower than the external mass transfer coefficient (k e) and more than 30 times lower than the intrabead diffusion coefficient (k i). So, the resistance due to surface adsorption is much higher than the other two resistances due to external diffusion and intrabead diffusion for the removal of Cr (VI) ion. Hence, the surface adsorption is acting as rate-limiting step for the whole removal process.
Aksu and Kutsal in 1998 [15] studied the biosorption of Cu (II) ions to the dried alga Cladophora sp. in a packed bed column reactor as a function of flow rate and inlet copper (II) concentration. They assumed that the algal particles were nonporous, spherical, and that only surface adsorption occurred. In the present study, a mutated bacterial strain B. cereus M1 16 immobilized in calcium alginate gel. Calcium alginate gel beads containing bacterial biomass are porous, spherical, and surface adsorption and solid-phase intrabead diffusion both take place. Aksu and Kutsal [15] showed adsorption rate increased slightly on increasing flow rates up to 4.45 ml/min. Increase in flow rate caused decrease in external mass transfer resistance but higher flow rates lowered the adsorption rates because of insufficient contact time for establishing adsorption equilibrium between biomass and inlet metal ions. They carried out the biosorption at pH 4.0 which was optimized in previous study in batch system where as we used metal solution at pH 3.0 which was optimized previously [35]. Aksu and Kutsal reported that external mass transfer coefficient is at least 20 times higher than adsorption rate constant. In their studies, it was also concluded that the surface adsorption step was acting as rate-limiting step. It may be concluded from our study that the surface adsorption phenomenon is acting as the rate-limiting step due to high resistance for removal of Cr (VI).
França et al. reported continuous biotreatment of copper-concentrated solutions by biosorption with Sargassum sp. The biomass of Sargassum in the bioreactors biosorbed the ionic copper sulfate contained in 63 l of copper sulfate solution, 72 l copper nitrate solution, all the solutions containing copper 500 mg/l. Effluents produced after biosorption presented copper concentration <0.05 mg/l [36].
Ksungur et al. reported biosorption of copper ions by caustic treated waste baker’s yeast biomass immobilized in calcium alginate using column reactor. The flow rate in the packed bed bioreactor was 2.35 ml/min and the inlet copper concentration in the feed was held constant at 95.5 mg/l (pH 4.0). The residence time of metal ions through the column was 11.3 min. At the initial phase of biosorption, calcium alginate gel with immobilized biosorbent removed 98.3% of copper [37].
Yu et al. in 2001 reported the removal of lead and copper by sawdust using packed bed column reactor. The operation capacity of sawdust of copper and lead in mixture was calculated. Same results were obtained using both the concentrations, viz., 50 and 100 mg/l. The capacity is 0.65 mg/g SD (0.02 meq/gSD) for copper and 1.1 mg/g SD (0.01 meq/gSD) for lead. The total operation capacity is 0.03 meq/gSD [38].
Conclusion
In the present study, Cr (VI) removal in a continuous flow packed bed column reactor with B. cereus M1 16 immobilized in calcium alginate was found to be highly effective. The immobilized microorganism beads were assumed to be porous and spherical. External diffusion of metal ions from bulk solution to bead surface, surface adsorption, and intrabead diffusion were assumed as main steps for overall removal of Cr (VI) from the solution. The process parameters of packed bed column such as inlet flow rate, bed height, and inlet metal concentration were very important and regulating the metal removal rates. At higher flow rates, the external mass transfer coefficients increased but it was observed that at higher flow rate, the percent removal rates decreased due to insufficient contact time for the establishment of equilibrium between bulk solution and beads. With increase in effective bed height, the uptake of Cr (VI) ion increased due to increase in amount of binding sites in the column. At higher inlet metal ion concentration, the rate of removal of metal ions also increased due to increase of the driving force between bulk solution and the immobilized beads. The external mass transfer coefficient, surface adsorption rate constant, and intrabead mass transfer coefficient predicted from the model give some ideas of kinetics and also controlling step for removal of Cr (VI). It was observed that surface adsorption rate constant was very low compare to other two mass transfer coefficient. So, the resistance of surface adsorption is acting as a controlling step. Further, all the kinetics parameters and controlling step information can be useful for process design and optimization of biosorption process.
- A col :
-
cross sectional area of the column (cm2)
- A p :
-
external surface area of per unit weight of immobilized bead (cm2/g)
- C 0 :
-
inlet metal ion concentration (mg/l)
- C S :
-
metal ion concentration adsorbed onto immobilized bead (mg/l)
- d m :
-
diameter of immobilized bead (cm)
- D e :
-
external diffusivity of metal ion solution (cm2/min)
- D i :
-
intra bead diffusivity (cm2/min)
- F :
-
flow rate of metal ion into packed bed column (ml/min)
- F N :
-
Faraday’s constant (coulomb/gequive)
- G :
-
adsorbent amount in packed bed (g)
- IBD:
-
intrabead diffusion
- L :
-
effective height of the packed bed column (cm)
- k ad :
-
surface adsorption (SA) rate constant (l/g/min)
- k aid :
-
removal rate constant by SA plus IBD (l/g/min)
- k e :
-
external mass transfer coefficient (cm/min)
- k i :
-
solid phase (intra bead) mass transfer coefficient (l/g/min)
- k o :
-
overall first order removal rate constant (l/g/min)
- K :
-
removal constant (l/g)
- M u :
-
amount of total metal uptake in the column (mg)
- M total :
-
amount of total metal ion entered to the column (mg)
- N Re :
-
Reynolds number
- N sc :
-
Schmidt number
- N sh :
-
Sherwood number
- q :
-
metal uptake at any time (mg/g)
- q e :
-
metal uptake at equilibrium (mg/g)
- r aid :
-
net metal ion removal rate in the column by SA and IBD at equilibrium (mg/g/min)
- r aid :
-
experimentally Cr (VI) removal rate (mg/g/min)
- r s :
-
external mass transfer rate (mg/g/min)
- R :
-
universal gas constant (J/mol/K)
- RM:
-
total removal percentage %
- SA:
-
surface adsorption
- T :
-
absolute temperature (K)
- ν sol :
-
linear velocity of solution in column (cm/min)
- z +, z − :
-
valences of cation and anion
- λ+, λ− :
-
cationic and anionic conductance at infinite dilution (mhos/gequive)
- ɛ :
-
void fraction in packed bed
- ϕ s :
-
sphericity of the immobilized bead
- τ :
-
residence time of solution in the column(min)
- ρ sol :
-
density of metal containing solution (g/ml)
- μ sol :
-
viscosity of metal containing solution (g/cm/min)
References
Tewari, N., Vasudevan, P., & Guha, B. K. (2005). Biochemical Engineering Journal, 23(2), 185–192. doi:10.1016/j.bej.2005.01.011.
Baruthio, F. (1992). Biological Trace Element Research, 32, 145–153. doi:10.1007/BF02784599.
EU. (1998). Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption (O.J. L 330/32, 5.12.98).
Nourbakhsh, M., Sag, Y., Ozer, D., Aksu, Z., Kutsal, T., & Caglar, A. (1994). Process Biochemistry, 29(1), 1–5. doi:10.1016/0032-9592(94)80052-9.
Veglio, F., & Beolchini, F. (1997). Hydrometallurgy, 44(7), 301–316. doi:10.1016/S0304-386X(96)00059-X.
Kratochvil, D., & Volesky, B. (1998). Trends in Biotechnology, 16(7), 291–300. doi:10.1016/S0167-7799(98)01218-9.
Volesky, B. (2001). Hydrometallurgy, 59(2), 203–216. doi:10.1016/S0304-386X(00)00160-2.
Mchale, A. P., & Mchale, S. (1994). Biotechnology Advances, 12(4), 647–652. doi:10.1016/0734-9750(94)90005-1.
Volesky, B., & Holan, Z. R. (1995). Biotechnology Progress, 11(3), 235–250. doi:10.1021/bp00033a001.
Trujillo, E. M., Sprinti, M., & Zhuang, H. (1995). In A. K. Senguptal (Ed.), Ion exchange technology: Advances in pollution control (pp. 225–272). Pennsylvania: Technomic.
Sag, Y., Tatar, B., & Kutsal, T. (2003). Bioresource Technology, 87(1), 27–33. doi:10.1016/S0960-8524(02)00210-9.
Kogej, A., & Pavko, A. (2001). Chemical and Biochemical Engineering Quarterly, 15(2), 75–79.
Beveridge, T. J., & Fyfe, W. S. (1985). Canadian Journal of Earth Sciences, 22, 1893–1898.
Tsezos, M., Hatzikioseyian, A., & Remoundaki, E. (2004). Proceedings of European Commission—Canmet Joint Workshop on Clean Production Technologies, Madrid, Spain, 30th September–2nd October, pp 279–292.
Aksu, Z., & Kutsal, T. (1998). Process Biochemistry, 33(1), 7–13. doi:10.1016/S0032-9592(97)00052-6.
Trevan, M. D. (1980). Immobilized enzymes, an introduction and applications in biotechnology. New York: Wiley.
Akhtar, N., Iqbal, J., & Iqbal, M. (2003). Letters in Applied Microbiology, 37(2), 149–253. doi:10.1046/j.1472-765X.2003.01366.x.
Goksungur, Y., Uren, S., & Guvenc, U. (2003). Turkish Journal of Biology, 27(1), 23–29.
Khosravi, M., Rakhshaee, R., & Ganji, M. T. (2005). Journal of Hazardous Materials, B127, 228–237. doi:10.1016/j.jhazmat.2005.07.023.
BRITE EURAM III BE95-1610/BRPR-CT96-0172. (1999). Removal and recovery of heavy metals from waste water by sand filters inoculated with metal biosorbing or bioprecipitating bacteria. Final technical report.
Kratochvil, D., & Volesky, B. (2000). Water Research, 34, 3186–3196. doi:10.1016/S0043-1354(00)00083-X.
Volesky, B., & Prasetyo, I. (1994). Biotechnology and Bioengineering, 43, 1010–1015. doi:10.1002/bit.260431103.
Figueira, M. M., Volesky, B., Azarian, K., & Ciminelli, V. S. T. (2000). Environmental Science & Technology, 34, 4320–4326. doi:10.1021/es001027l.
Hatzikioseyian, A., Tsezos, M., & Mavituna, F. (2001). Hydrometallurgy, 59, 395–406. doi:10.1016/S0304-386X(00)00169-9.
Low, K. S., Lee, C. K., & Ng, A. Y. (1999). Bioresource Technology, 68, 205–208. doi:10.1016/S0960-8524(98)00128-X.
Zhao, M., & Duncan, J. R. (1997). Resource and Environmental Biotechnology, 2, 51–64.
Prouzet, E., Khani, Z., Bertrand, M., Tokumoto, M., Ferreol, V. G., & Tranchant, J. F. (2006). Microporous and Mesoporous Materials, 96, 369–375. doi:10.1016/j.micromeso.2006.07.011.
Idris, A., & Suzana, W. (2006). Process Biochemistry, 41, 1117–1123. doi:10.1016/j.procbio.2005.12.002.
Weber Jr, W. J., McGinley, P. M., & Katz, L. E. (1991). Water Research, 25, 499–528. doi:10.1016/0043-1354(91)90125-A.
Wakao, N., & Funazkri, T. (1978). Chemical Engineering Science, 33(10), 1375–1384. doi:10.1016/0009-2509(78)85120-3.
Longsworth, L. G. (1972). In D. W. Gray (Ed.), Diffusion in liquids: American institute of physics hand book. New York: McGraw-Hill.
Urano, K., & Tachikawa, H. (1991). Industrial & Engineering Chemistry Research, 30, 1897–1899. doi:10.1021/ie00056a033.
Treybal, R. E. (1984). In Mass transfer operation (p. 603, 3rd ed.). Auckland: McGraw-Hill.
Bera, D., Ray, L., & Chattopadhyay, P. (2007). Journal of Hazardous Substance Research, 7, 1–19.
Bera, D., Chattopadhyay, P., & Ray, L. (2006). Journal for Hazardous Substance Research, 6(2), 1–23.
França, F. P., Padilha, F. P., & Costa, A. C. A. (2006). Applied Biochemistry and Biotechnology, 128(1), 23–32. doi:10.1385/ABAB:128:1:023.
Ksungur, Y. G., Ren, S., & Ven, U. G. (2003). Turkish Journal of Biology, 27, 23–29.
Yu, B., Zhang, Y., Shukla, A., Shukla, S. S., & Dorris, K. L. (2001). Journal of Hazardous Materials, B84(1), 83–94. doi:10.1016/S0304-3894(01)00198-4.
Acknowledgment
The authors gratefully acknowledge the financial support from University Grant Commission (UGC) India in carrying out the research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maiti, S.K., Bera, D., Chattopadhyay, P. et al. Determination of Kinetic Parameters in the Biosorption of Cr (VI) on Immobilized Bacillus cereus M1 16 in a Continuous Packed Bed Column Reactor. Appl Biochem Biotechnol 159, 488–504 (2009). https://doi.org/10.1007/s12010-008-8519-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8519-2