Abstract
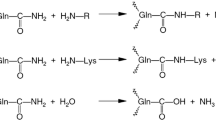
Transglutaminase (TGase) is a multifunctional enzyme vital for many physiologic processes, such as cell differentiation, tissue regeneration, and plant pathogenicity. The acyl transfer function of the enzyme can activate primary amines and, consequently, attach them onto a peptidyl glutamine, a reaction important for various in vivo and in vitro protein crosslinking and modification processes. To understand better the structure-function relationship of the enzyme and to develop it further as an industrial biocatalyst, we studied TGase secreted by several Streptomyces species and Phytophthora cactorum. We purified the enzyme from S. lydicus, S. platensis, S. nigrescens, S. cinnamoneus, and S. hachijoensis. The pH and temperature profiles of S. lydicus, S. platensis, and S. nigrescens TGases were determined. The specificity of S. lydicus TGase toward its acyl-accepting amine substrates was characterized. Correlation of the electronic and steric features of the substrates with their reactivity supported the mechanism previously proposed for Streptomyces mobaraensis TGase.
Similar content being viewed by others
References
Facchiano, F. and Facchiano, A. (2005), Prog. Exp. Tumor Res. 38, 37–57.
Yokoyama, K., Nio, N., and Kikuchi, Y. (2004), Appl. Microbiol. Biotechnol. 64, 447–454.
Lorand, L. and Graham, R. M. (2003), Nat. Rev. Mol. Cell Biol. 4, 140–156.
Liu, S., Cerione, R. A., and Clardy, J. (2002), Proc. Natl. Acad. Sci. USA 99, 2743–2747.
Bech, L., Norrevang, I. A., Halkier, T., Rasmussen, G., Schaffer, T., Andersen, J. T. and Schafer, T. (1996), International patent WO9606931-A1.
Bech, L., Rasmussen, G., Halkier, T., Okada, M., Andersen, L. N., and Sandal, T. (2002), US patent US6428933-B1.
Leblanc, A., Gravel, C., Labelle, J., and Keillor, J. W. (2001), Biochemistry 40, 8335–8342.
Umezawa, Y., Ohtsuka, T., Yokoyama, K., and Nio, N. (2002), Food Sci. Technol. Res. 8, 113–118.
Kashiwagi, T., Yokoyama, K., Ishikawa, K., Ono, K., Ejima, D., Matsui, H., and Suzuki, E. (2002), J. Biol. Chem. 277, 44,252–44,260.
Folk, J. E. (1983), Adv. Enzymol. Relat. Areas Mol. Biol. 54, 1–56.
Lorand, L., Parameswaran, K. N., Stenberg, P., et al. (1979), Biochemistry 18, 1756–1765.
Ohtsuka, T., Sawa, A., Kawabata, R., Nio, N., and Motoki, M. (2000), J. Agric. Food Chem. 48, 6230–6233.
Castro, E. A. and Ureta C. (1989) J. Org. Chem. 54, 2153–2159.
Song, H. B., Choi, M. H., Koo, I. S., Oh, H. K., and Lee, I. (2003), Bull. Korean Chem. Soc. 24, 91–94.
Hansch, C. H. (1979), Substituent Constants for Correlation Analysis in Chemistry and Biology, John Wiley, New York.
Perrin, D. D. (1981), pK a Prediction for Organic Acids and Bases, Chapman &Hall, New York.
Andou, H., Matsuura, A., and Hirose, H. (1993), US patent 5,252,469-A1.
Pedersen, L. C., Yee, V. C., Bishop, P. D., Le Trong, I., Teller, D. C., and Stenkamp, R. E. (1994), Protein Sci. 3, 1131–1135.
Nury, S., Meunier, J.-C., and Mouranche, A. (1989), Eur. J. Biochem. 180, 161–166.
Folk, J. E. and Cole, P. W. (1966), Biochim. Biophys. Acta 122, 244–264.
Brunner, F., Rosahl, S., Lee, J., et al. (2002), EMBO J. 21, 6681–6688.
Grosclaude, F., Mahe, M. F., and Ribadeau-Dumas, B. (1973), Eur. J. Biochem. 40, 323, 324.
Gorman, J. J. and Folk, J. E. (1984), J. Biol. Chem. 259, 9007–9010.
Kahlem, P., Terré, C., Green, H., and Djian, P. (1996), Proc. Natl. Acad. Sci. USA 93, 14,580–14,585.
Hu, B. and Messersmith, P. B. (2003), J. Am. Chem. Soc. 125, 14,298, 14,299.
Griffin, M., Casadio, R., and Bergamini, C. M. (2002), Biochem. J. 368, 377–396.
Makarova, K. S., Aravind, L., and Koonin, E. V. (1999), Protein Sci. 8, 1714–1719.
Nielsen, P. M. (1995), Food Biotechnol. 9, 119–156.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Langston, J., Blinkovsky, A., Byun, T. et al. Substrate specificity of Streptomyces transglutaminases. Appl Biochem Biotechnol 136, 291–308 (2007). https://doi.org/10.1007/s12010-007-9027-5
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s12010-007-9027-5