Abstract
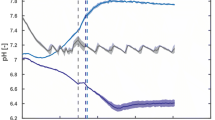
A simple automated glucose feeding strategy based on pH control was developed to produce high-cell-density fed-batch fermentation. In this strategy, the pH control scheme utilized an acidified concentrated glucose solution to lower the pH. The frequency of glucose addition to the fermentor is determined by the culture’s growth kinetics. To demonstrate the effectiveness of the coupled pH and glucose control strategy in biomass and/or secondary metabolite production, several fed-batch fermentations of indigenous Escherichia coli and recombinant E. coli were carried out. Both strains produced biomass with optical density of greater than 40 at 600 nm. We also tested the glucose control strategy using two types of pH controller: a less sophisticated portable pH controller and a more sophisticated online proportional-integral-derivative (PID) controller. Our control strategy was successfully applied with both controllers, although better control was observed using the PID controller. We have successfully demonstrated that a glucose feeding strategy based on a simple pH control scheme to indirectly control the glucose concentration can be easily achieved and adapted to conventional bioreactors in the absence of online glucose measurement and control.





Similar content being viewed by others
References
Luli, G. W., & Strohl, W. R. (1990). Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch and fed-batch fermentations. Applied and Environmental Microbiology, 56(4), 1004–1011.
Akesson, M., Hagander, P., & Axelsson, J. P. (2001). Avoiding acetate accumulation in Escherichia coli cultures using feedback control of glucose feeding. Biotechnology and Bioengineering, 73(3), 223–230.
Hewitt, C. J., Caron, G. N., Axelsson, B., McFarlane, C. M., & Nienow, A. W. (2000). Studies related to the scale-up of high cell density E. coli fed-batch fermentations using multiparameter flow cytometry: Effect of a changing microenvironment with respect to glucose and dissolved oxygen concentration. Biotechnology and Bioengineering, 70(4), 381–390.
Xu, B., Jahic, M., & Enfors, S-O. (1999). Modeling of overflow metabolism in batch and fed-batch cultures of Escherichia coli. Biotechnology Progress, 15, 81–90.
George, S., Larsson, G., & Enfors, S.-O. (1993). A scale-down two compartment reactor with controlled substrate oscillations: Metabolic response of Saccharomyces cerevisiae. Bioprocess Engineering, 9, 249–257.
Jobe, A. M., Herwig, C., Surzyn, M., Walker, B., Marison, I., & Stockar, U. V. (2003). Generally applicable fed-batch culture concept based on the detection of metabolic state by on-line balancing. Biotechnology and Bioengineering, 82(6), 627–639.
Panda, A. K., Khan, R. K., Appa Rao, K. B. C., & Totey, S. M. (1999). Kinetics of inclusion body production in batch and high cell density fed-batch culture of Escherichia coli expressing ovine growth hormone. Journal of Biotechnology, 75, 161–172.
Hellmuth, K., Korz, D. J., Sanders, E. A., & Deckwer, W.-D. (1994). Effect of growth rate on stability and gene expression of recombinant plasmids during continuous and high cell density cultivation of Escherichia coli TG1. Journal of Biotechnology, 32, 289–298.
Arndt, M., & Hitzmann, B. (2004). Kalman filter based glucose control at small set points during fed-batch cultivation of Saccharomyces cerevisiae. Biotechnology Progress, 20, 377–383.
Akesson, M., Karlsson, E. N., Hagander, P., Axelsson, J. P., & Tocaj, A. (1999). On-line detection of acetate formation in Escherichia coli cultures using dissolved oxygen responses to feed transients. Biotechnology and Bioengineering, 64(5), 590–598.
Andersen, D. C., Swartz, J., Ryll, T., Lin, N., & Snedecor, B. (2001). Metabolic oscillations in an E. coli fermentation. Biotechnology and Bioengineering, 75(2), 212–218.
Kim, B. S., Lee, S. C., Lee, S. Y., Chang, H. N., Chang, Y. K., & Woo, S. I. (1994). Production of poly(3-hydroxybutyric acid) by fed-batch culture of Alcaligenes eutrophus with glucose concentration control. Biotechnology and Bioengineering, 43, 892–898.
Kleman, G. L., Chalmers, J. J., Luli, G. W., & Strohl, W. R. (1991). A predictive and feedback control algorithm maintains a constant glucose concentration in fed-batch fermentations. Applied and Environmental Microbiology, 57(4), 910–917.
Wang, F., & Lee, S. (1998). High cell density culture of metabolically engineered Escherichia coli for the production of poly-(3-hydroxybutyrate) in a defined medium. Biotechnology and Bioengineering, 58, 325–328.
Sanden, A. M., Prytz, I., Tubulekas, I., Forberg, C., Le, H., Hektor, A., et al. (2002). Limiting factors in Escherichia coli fed-batch production of recombinant proteins. Biotechnology and Bioengineering, 81(2), 158–166.
Liu, H. (2003). The identification of differences during GFP production and purification due to the fusion of IMAC tails and other implications through metabolic flux analysis. Ph.D. Dissertation, University of Arkansas, Fayetteville, Arkansas, 165p.
Ting, T. (2002). Development of a bacterial tracer for water quality studies in mantled karst basin using indigenous Escherichia coli labeled with europium. Master Thesis, University of Arkansas, Fayetteville, Arkansas, 105p.
van Wegen, R. J., Lee, S., & Middelberg, A. P. J. (2001). Metabolic and kinetic analysis of poly-(3-hydroxybutyrate) production by recombinant Escherichia coli. Biotechnology and Bioengineering, 74(1), 70–80.
Acknowledgement
This work was supported in part by a grant (EAR-0207793) from the National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ting, TE., Thoma, G.J., Beitle, R.R. et al. A Simple Substrate Feeding Strategy using a pH Control Trigger in Fed-Batch Fermentation. Appl Biochem Biotechnol 149, 89–98 (2008). https://doi.org/10.1007/s12010-007-8089-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-007-8089-8