Abstract
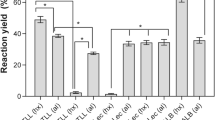
Lipase immobilization offers unique advantages in terms of better process control, enhanced stability, predictable decay rates and improved economics. This work evaluated the immobilization of a highly active Yarrowia lipolytica lipase (YLL) by physical adsorption and covalent attachment. The enzyme was adsorbed on octyl–agarose and octadecyl–sepabeads supports by hydrophobic adsorption at low ionic strength and on MANAE–agarose support by ionic adsorption. CNBr–agarose was used as support for the covalent attachment immobilization. Immobilization yields of 71, 90 and 97% were obtained when Y. lipolytica lipase was immobilized into octyl–agarose, octadecyl–sepabeads and MANAE–agarose, respectively. However, the activity retention was lower (34% for octyl–agarose, 50% for octadecyl–sepabeads and 61% for MANAE–agarose), indicating that the immobilized lipase lost activity during immobilization procedures. Furthermore, immobilization by covalent attachment led to complete enzyme inactivation. Thermal deactivation was studied at a temperature range from 25 to 45°C and pH varying from 5.0 to 9.0 and revealed that the hydrophobic adsorption on octadecyl–sepabeads produced an appreciable stabilization of the biocatalyst. The octadecyl–sepabeads biocatalyst was almost tenfold more stable than free lipase, and its thermal deactivation profile was also modified. On the other hand, the Y. lipolytica lipase immobilized on octyl–agarose and MANAE–agarose supports presented low stability, even less than the free enzyme.


Similar content being viewed by others
References
Cammarota, M. C., & Freire, D. M. G. (2006). Bioresource Technology, 97, 2195–2210.
Gotor-Fenandez, V., Brieva, R., & Gotor, V. (2006). Journal Molecular Catalysis B: Enzymatic, 40, 111–120.
Mateo, C., Palomo, J. M., Fernandez-Lorente, G., Guisán, J. M., & Fernandez-Lafuente, R. (2007). Enzyme and Microbial Technology, 40, 1451–1463.
Koeller, K. M., & Wong, C.-H. (2001). Nature, 409, 232–240.
Bevilaqua, J. B., Lima, L. M., Cunha, A. G., Barreiro, E. J., Alves, T. L. M., & Paiva, L. M. C., et al. (2005). Applied Biochemistry and Biotechnology, 121, 117–128.
Shibatane, T., Omori, K., Akatsuka, H., Kawai, E., & Matsumae, H. (2000). Journal of Molecular Catalysis B: Enzymatic, 10, 141–149.
Mosbach, K. (1971). Science American, 224, 26–32.
Palomo, J. M., Segura, R. L., Fernandez-Lorente, G., Guisán, J. M., & Fernandez-Lafuente, R. (2007). Enzyme and Microbial Technology, 40, 704–707.
Villeneuve, P., Muderhwa, J. M., Graille, J., & Haas, M. J. (2000). Journal of Molecular Catalysis B: enzymatic, 9, 113–148.
Gupta, M. N. (1991). Biotechnology Applied Biochemistry, 14, 1–11.
Klibanov, A. M. (1982). Advanced Applied Microbiology, 29, 1–28.
Klibanov, A. M. (1983). Biochemical Society Transactions, 11, 19–20.
Mozhaev, V. V., Melik-Nubarov, N. S., Sergeeva, M. V., Sikrnis, V., & Martinek, K. (1990). Biocatalysis, 3, 179–87.
Nanalov, R. J., Kamboure, M. S., & Emanuiloda, E. I. (1993). Biotechnology Applied Biochemistry, 18, 409–416.
Jaeger, K. E., Dijkstra, B. W., & Reetz, M. T. (1999). Annual Review of Microbiology, 53, 315–351.
Aloulou, A., Rodriguez, J. A., Fernandez, S., Osterhout, D., Puccinelli, D., & Carriere, F. (2006). Biochimica et Biophisica Acta, 1761, 995–1013.
Fernandez-Lafuente, R., Armisén, P., Sabuquillo, P., Fernández-Lorente, G., & Guisán, J. M. (1998). Chemistry and Physics of Lipids, 93, 185–197.
Palomo, J. M., Muñoz, G., Fernández-Lorente, G., Mateo, C., Fernández-Lafuente, R., & Guisán, J. M. (2002). Journal of Molecular Catalysis B: Enzymatic, 19, 279–286.
Lowry, O., Rosenbrough, M., Farr, A., & Randall, R. (1951). Journal of Biological Chemistry, 193, 265–275.
Destain, J., Roblain, D., & Thonart, P. (1997). Biotechnology Letters, 19, 105–107.
Cabrera, Z., Palomo, J. M., Fernandez-Lorente, G., Fernandez-Lafuente, R., & Guisan, J. M. (2007). Enzyme and Microbial Technology, 40, 1280–1285.
Fernandez-Lafuente, R., Rosell, C. M., Rodriguez, V., Santana, C., Soler, G., & Batisda, A., et al. (1993). Enzyme and Microbial Technology, 15, 546–550.
Petkar, M., Lali, A., Caimi, P., & Daminati, M. (2006). Journal of Molecular Catalysis B: enzymatic, 9, 83–90.
Palomo, J. M., Segura, R. L., Fernandez-Lorente, G., Pernas, M., Rua, M. L., & Guisán, J. M., et al. (2004). Biotechnology Progress, 20, 630–635.
Oliveira, D., Feihrmann, A. C., Dariva, C., Cunha, A. G., Bevilaqua, J. V., & Destain, J., et al. (2006). Journal of Molecular Catalysis B: Enzymatic, 39, 117–123.
Lipase Engineering Database is available as http://www.led.uni-stuttgardt.de/.
Wilson, L., Palomo, J. M., Fernández-Lorente, G., Illanes, A., Guisan, J. M., & Fernandez-Lafuente, R. (2006). Enzyme and Microbial Technology, 38, 975–980.
Palomo, J. M., Fuentes, M., Fernández-Lorente, G., Mateo, C., Guisán, J. M., & Fernández-Lafuente, R. (2003). Biomacromolecules, 4, 1–6.
Acknowledgements
Financial support was gratefully received from PETROBRÁS, FUJB, FAPERJ and CAPES. The authors are also grateful to Prof Rodrigo Volcan Almeida for his contribution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cunha, A.G., Fernández-Lorente, G., Bevilaqua, J.V. et al. Immobilization of Yarrowia lipolytica Lipase—a Comparison of Stability of Physical Adsorption and Covalent Attachment Techniques. Appl Biochem Biotechnol 146, 49–56 (2008). https://doi.org/10.1007/s12010-007-8073-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-007-8073-3