Abstract
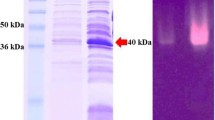
Wild type (WT) DNA sequence, which encoded a mature β-mannanase of Aspergillus sulphureus, composed of 1,152 nucleotides (nt), was amplified from pUCm-T-mann by polymerase chain reaction (PCR). Based on this DNA fragment, mutants designated as E206G and E314G were constructed by overextension PCR (OE-PCR). Glutamic acids of the 206th and 314th sites in the amino acid sequence of β-mannanase were separately replaced by glycine in these two mutants. The WT and mutant genes were ligated into prokaryotic vector pET-28a (+) and transformed into the Escherichia coli BL21 strain, respectively. The recombinant enzyme proteins were expressed by IPTG induction and detected by Western blot. The recombinant proteins purified with Ni-NTA column were dialyzed to correctly refold. The WT recombinant β-mannanase showed optimal activity at 50 °C and pH 2.4. The kinetic parameters of K m and V max for purified β-mannanase were 1.38 mg/ml and 72.99 U/mg, respectively. However, the mutant proteins did not show any activity. It was demonstrated that E206 and E314 were the catalytic residues of β-mannanase.




Similar content being viewed by others
References
Matheson, N. K., & McCleary, B. V. (1985). In G. O. Aspinall (Ed.), The Polysachharides (vol. 3, p. 1). New York: Academic.
McCleary, B. V., & Matheson, N. K. (1986). Enzymic analysis of polysaccharide structure. Advances in Carbohydrate Chemistry and Biochemistry, 44, 147–276.
Henrissat, B., & Bairoch, A. (1993). New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochemical Journal, 293, 781–788.
Stålbrand, H., Saloheimo, A., Vehmaanperä, J., Henrissat, B., & Penttilä, M. (1995). Cloning and expression in Saccharomyces cerevisiae of a Trichoderma reesei β-mannanase gene containing a cellulose binding domain. Applied and Environmental Microbiology, 61, 1090–1097.
Chen, X. L., Cao, Y. H., Ding, Y. H., Lu, W. Q., & Li, D. F. (2007). Cloning, functional expression and characterization of Aspergillus sulphureus β-mannanase in Pichia pastoris. Journal of Biotechnology, 128, 452–461.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: A laboratory manual (2nd ed., p. 808). Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press.
Lu, W. Q., Li, D. F., & Wu, Y. B. (2003). Influence of water activity and temperature on xylanase biosynthesis in pilot-scale solidstate fermentation by Aspergillus sulphureus. Enzyme and Microbial Technology, 32, 305–311.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugars. Analytical Chemistry, 31, 426–428.
Acknowledgement
This research was supported by funds from National Natural Science Foundation of People’s Republic of China (No. 30600433) and National Basic Research Program (2004CB117503).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, X., Lu, W., Cao, Y. et al. Prokaryotic Expression, Purification and Characterization of Aspergillus sulphureus β-Mannanase and Site-Directed Mutagenesis of the Catalytic Residues. Appl Biochem Biotechnol 149, 139–144 (2008). https://doi.org/10.1007/s12010-007-8037-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-007-8037-7