Abstract
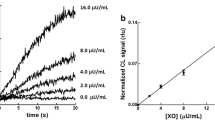
A highly sensitive method for measuring the activity of the enzyme diamine oxidase (DAO) independent of the type of substrate is described. The principle of the assay is to determine the amount hydrogen peroxide generated as a reaction product during oxidation of diamines by DAO. PSatto™, a highly sensitive luminescence reagent, was used to generate a signal depending on the hydrogen peroxide concentration based on the action of horseradish peroxidase. DAO is specifically captured from a sample by an antibody immobilized to microwell plates, and the substrate is added to the bound enzyme. Various diamines were used as substrates; the peroxide produced is directly proportional to the amount of DAO bound to the specific antibodies. With this very sensitive method, it is possible to detect pmol amounts of generated hydrogen peroxide in plasma matrix corresponding to the biological activity of DAO.










Similar content being viewed by others
References
Buffoni, F. (1966). Pharmacological Reviews, 18, 1163–1199.
Buffoni, F., & Ignesti, G. (2003). Inflammopharmacology, 11, 203–209.
Aschenbach, J. R., Schwelberger, H. G., Ahrens, F., Fürll, B., & Gäbel, G. (2006). Scandinavian Journal of Gastroenterology, 41, 712–719.
Maintz, L., & Novak, N. (2007). American Journal of Clinical Nutrition, 85, 1185–1196.
Sattler, J., Lorenz, W., Kubo, K., Schmal, A., Sauer, S., & Luben, L. (1989). Journal of Chromatography B, Biomedical Sciences and Applications, 27, 212–214.
Sattler, J., Hafer, D., Klotter, H. J., Lorenz, W., & Wagner, P. K. (1988). Agents Actions, 23, 361–365.
Cubria, C., Alvarez-Bujidos, M., Negro, A., Balana-Fouce, R., & Ordonez, D. (1993). Comparative Biochemistry and Physiology C, 105, 251–254.
Schwelberger, H. G., & Bodner, E. (1997). Biochimica et Biophysica Acta, 1340, 152–164.
Kluetz, D., & Schmidt, P. G. (1977). Biochemical and Biophysical Research Communications, 76, 40–45.
Rinaldi, A., Vecchini, P., & Floris, G. (1982). Preparative Biochemistry, 12, 11–28.
Wilfingseder, D., & Schwelberger, H. G. (2000). Journal of Chromatography B, Biomedical Sciences and Applications, 737, 161–166.
Shah, M. A., & Ali, R. (1988). Biochemical Journal, 253, 103–107.
Biebl, M., Klocker, J., Perkmann, R., Kolbitsch, C., Klingler, P., Drasche, A., et al. (2002). Inflammation Research, 51(1), 93–94.
Ignesti, G. (2003). Journal of Enzyme Inhibition and Medicinal Chemistry, 18, 463–473.
Klaus, A., Weiss, H., Nguyen, J. H., Margreiter, R., Obrist, P., & Schwelberger, H. G. (2003). Transplant International, 16, 474–479.
Sattler, J., Hafner, D., Klotter, H. J., Lorenz, W., & Wagner, P. K. (1988). Agents Actions, 23, 361–365.
Sattler, J., Lorenz, W., Kubo, K., Schmal, A., Sauer, S., & Luben L. (1989). Agents Actions, 27, 212–214.
Jarisch, R., & Wantke, F. (1996). International Archives of Allergy and Immunology, 110, 7–12.
Nag, S., Saha, K., & Choudhuri, M. A. (2000). Plant Science, 157, 157–163.
Takagi, K., Nakao, M., Ogura, Y., Nabeshima, T., & Kunii, A. (1994). Clinica Chimica Acta, 226, 67–75.
Houen, G. (1999). Acta Pathologica, Microbiologica et Immunologica Scandinavica, Supplementum, 96, 1–46.
Ionescu, G., & Kiehl, R. (1988). Allergy, 43, 318–319.
Tufvesson G., & Tryding, N. (1969). Scandinavian Journal of Clinical and Laboratory Investigation, 24, 163–168.
Mayer, I., Missbichler, A., Wantke, F., Focke, M., Reichl, H., Winter, M., et al (2005). Allergologie, 28, 1–8.
Bachrach, U. (1985). In: Mondovi (Ed.), Structure and Functions of Amine Oxidase (pp. 5–20). Boca Raton: CRC Press.
Bradsley, W. G., Crabbe, M. J. C., & Scott I. V. (1974). Biochemical Journal, 139, 169–181.
Kufner, M. A., Ulrich, P., Raithel, M., & Schwelberger, H. G. (2001). Inflammation Research, 50(2), 96–97.
Sasaki, A., Tsujikawa, T., Fujiyama, Y., & Bama, T. (2001). Journal of Gastroenterology and Hepatology, 16(9), 986–990.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mayer, I., Pittner, F., Hermann, M. et al. Highly Sensitive Determination of DAO Activity by Oxidation of a Luminescence Reagent. Appl Biochem Biotechnol 143, 164–175 (2007). https://doi.org/10.1007/s12010-007-8023-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-007-8023-0