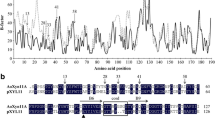
Abstract
The amino acid sequences of xylanase B (XlnB) and xylanase C (XlnC) from Streptomyces lividans show significant homology. However, the temperature optima and stabilities of the two enzymes are quite different. XlnB exhibits an optimum temperature of 40 °C and retains 50% of its maximum activity at 43 °C, whereas the corresponding values for XlnC are 60 and 70 °C. To analyze these properties further, as well as to study the effect of the exchange of homologous segments in the C-terminal region, four chimeras designated as BSC, BFC, CSB, and CFB were constructed by substituting segments from the C-terminal homologous region of XlnB gene with that of XlnC and in turn substituting XlnC gene with that of XlnB. The purified chimeric enzymes were characterized with respect to pH/temperature activity, stability, and kinetic parameters. Most of enzymatic properties of chimeras were admixtures of those of the two parents. The chimeric enzymes were optimally active at 45–55 °C and pH 7.0. Both K m and k cat values of chimeric enzymes for p-nitrophenyl-β-d-cellobioside were admixtures of both parental enzymes, except that the k cat value of chimeric BFC (2.79 s−1) was higher than that of parental XlnC (1.99 s−1). Notably, thermal stability of chimeric BSC and BFC was increased by 25 and 13 °C separately, as compared to one of parental XlnB, whereas the thermal stability of chimeric CSB and CFB was decreased by 23 and 21 °C, respectively, as compared to another parental XlnC. These results suggest that homologous C-terminal region in S. lividans GH11 xylanase appears to play an important role in determining enzyme characteristics, and exchanging of different segments of gene in this region might significantly alter or improve the enzymatic properties such as thermal stability.




Similar content being viewed by others
Abbreviations
- GH:
-
glycoside hydrolase
- XlnB:
-
Streptomyces lividans xylanase B
- XlnC:
-
Streptomyces lividans xylanase C
- PNP-G2 :
-
p-nitrophenyl-β-d-cellobioside
- RBB-xylan:
-
4-O-methyl-d-glucurono-d-xylan-Remazol Brilliant Blue R
References
Gilkes, N. R., Henrissat, B., Kilburn, D. G., Miller, R. C. Jr., & Warren, R. A. J. (1991). Microbiological Reviews, 55, 303–315.
Henrissat, B., & Bairoch, A. (1993). Biochemical Journal, 293, 781–788.
Polizeli, M. L., Rizzattim, A. C., Monti, R., Terenzi, H. F., Jorge, J. A., & Amorim, D. S. (2005). Applied Microbiology and Biotechnology, 67, 577–591.
Shareck, F., Roy, C., Yaguchi, M., Morosoli, R., & Kluepfel, D. (1991). Gene, 107, 75–82.
Biely, P., Kluepfel, D., Morosoli, R., & Shareck, F. (1993). Biochimica et Biophysica Acta, 1162, 246–254.
Sa-Pereira, P., Paveia, H., Costa-Ferreira, M., & Aires-Barros, M. (2003). Molecular Biotechnology, 24, 257–281.
Giannotta, F., Georis, J., Rigali, S., Virolle, M. J., & Dusart, J. (2003). Molecular Genetics and Genomics, 270, 337–346.
Tahir, T. A., Berrin, J. G., Flatman, R., Roussel, A., Roepstorff, P., Williamson, G., et al. (2002). Journal of Biological Chemistry, 277, 44035–44043.
van der Veen, B. A., Potocki-Veronese, G., Albenne, C., Joucla, G., Monsan, P., & Remaud-Simeon, M. (2004). FEBS Letters, 560, 91–97.
Rowe, L. A., Geddie, M. L., Alexander, O. B., & Matsumura, I. (2003). Journal of Molecular Biology, 332, 851–860.
Kim, Y. W., Choi, J. H., Kim, J. W., Park, C., Kim, J. W., Cha, H., et al. (2003). Applied and Environmental Microbiology, 69, 4866–4874.
Wang, J. D., Herman, C., Tipton, K. A., Gross, C. A., & Weissman, J. S. (2002). Cell, 111, 1027–1039.
Biely, P., Mislovicova, D., & Toman, R. (1988). Method in Enzymology, 160, 536–541.
Lawson, S. L., Wakarchuk, W. W., & Withers, S. G. (1996). Biochemistry, 35, 10110–10118.
Somogyi, M. (1952). Journal of Biological Chemistry, 195, 19–23
Singh, A., & Hayashi, K. (1995). Journal of Biological Chemistry, 270, 21928–21933.
Ahsan, M. M., Kaneko, S., Wang, Q., Yura, K., Go, M., & Hayash, K. (2001). Enzyme and Microbial Technology, 28, 8–15.
Singh, A., Hayashi, K., Hoa, T. T., Kashiwagi, Y., & Tokuyasu, K. (1995). Biochemical Journal, 305, 715–719.
Nosoh, Y., & Sekiguchi, T. (1990). Trends Biotechnology, 8, 16–20.
Garnier, J., Osguthorpe, D. J., & Robson, B. (1978). Journal of Molecular Biology, 120, 97–120.
Henrissat, B., Raimbound, E., Tran, V., & Mornon, J. P. (1990). Computer Applications in the Biosciences, 6, 3–5.
Gaboriaud, C., Bissery, V., Benchetrit, T., & Mornon, J. P. (1987). FEBS Letters, 224, 149–155.
Numata, K., Muro, M., Akutu, N., Nosoh, Y., Yamagishi, A., & Oshima, T. (1995). Protein Engineering, 8, 39–43.
Acknowledgments
We thank Ms. Ying Li for the assistance with the DNA sequence analysis and chimeric enzymes construction. This work was sponsored by Shanghai Pujiang Program to Qin Wang.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Q., Xia, T. Importance of C-Terminal Region for Thermostability of GH11 Xylanase from Streptomyces lividans . Appl Biochem Biotechnol 144, 273–282 (2008). https://doi.org/10.1007/s12010-007-8016-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-007-8016-z