Abstract
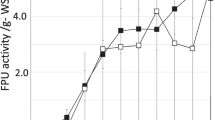
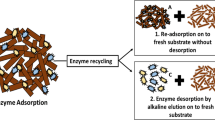
Cellulase distribution between residual substrate and supernatant in the process of enzymatic hydrolysis of steam-exploded wheat straw was investigated. Subsequently, a novel stepwise recovery strategy with three successive steps was adopted to recover cellulase adsorbed to the residual substrate. The results showed that cellulase protein in the supernatant increased as the hydrolysis time increased. When hydrolysis ended, the cellulase remaining on the residual substrate accounted for 33–42% of the original added cellulase according to the different cellulase loading. To obtain the maximum cellulase recovery rate, the residual substrate was dealt with in three successive steps: washed with sodium acetate buffer (step 1), shaken with sodium acetate buffer (step 2), and then treated with 0.0015 mol/L, pH 10 Ca(OH)2 (step 3). The total cellulase protein recovered by the three steps reached 96.70–98.14%. The enzyme activity of cellulase recovered by the first two steps was kept well. The ratios of the specific activity between the recovered cellulase and the original were 89–96%, which was by far higher than that using step 3 (the value was 48% ∼ 56%).




Similar content being viewed by others
References
Wilke, C. R., Yang, R. D., & von Stocker, V. (1976). Biotechnology and Bioengineering Symposium, 6, 155–175.
Zacchi, G., Skoog, K., & Hahn-Hagerdal, B. (1988). Biotechnology and Bioengineering, 32, 460–466.
Lee, Y. H., & Fan, L. T. (1983). Biotechnology and Bioengineering, 25, 939–966.
Ooshima, H., Burns, D. S., & Converse, A. O. (1990). Biotechnology and Bioengineering, 36, 446–452.
Lee, D., Yu, A. H. C., & Saddler, J. N. (1995). Biotechnology and Bioengineering, 45, 328–336.
Ramos, L. P., Breuil, C., & Saddler, J. N. (1993). Enzyme and Microbial Technology, 15, 19–25.
Mandels, M., Kostick, J., & Parizel, R. (1971). J. Polymer Sci. Part C, 36, 445–459.
Rao, M., Seeta, R., & Deshpande, V. (1982). Biotechnology and Bioengineering, 25, 1863–1871.
Otter, D. E., Munro, P. A., Scott, G. K., & Geddes, R. (1989). Biotechnology and Bioengineering, 34, 291–298.
Deshpande, M. V., & Eriksson, K. E. (1984). Enzyme and Microbial Technology, 6, 338–340.
Sinitsyn, A. P., Bungay, M. L., Clesceri, L. S., & Bungay, H. R. (1983). Applied Biochemistry and Biotechnology, 8, 25–29.
Chen, H. Z. (1998). PhD thesis, Institute of Chemical Metallurgy, Chinese Academy of Sciences, Beijing, China.
Chen, H. Z., Xu, J., & Li, Z. H. (2005). Biochemical Engineering Journal, 23, 117–122.
Miller, G. L. (1959). Analytical Chemistry, 31(3), 426–428.
Ghose, T. K. (1987). Pure and Applied Chemistry, 59, 257–268.
Acknowledgments
Financial support for this study was provided by National Basic Research Program of China (2004CB719700) and the Knowledge Innovation Program of Chinese Academy of Sciences (KJCXZ-SW-206-2).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, J., Chen, H. A Novel Stepwise Recovery Strategy of Cellulase Adsorbed to the Residual Substrate after Hydrolysis of Steam Exploded Wheat Straw. Appl Biochem Biotechnol 143, 93–100 (2007). https://doi.org/10.1007/s12010-007-0031-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-007-0031-6