Abstract
Background
CT pulmonary angiography (CTPA) has become widely adopted to detect pulmonary embolism (PE) after total joint arthroplasty (TJA). CTPA is a sensitive tool, which has the ability to detect emboli that may be clinically insignificant. This may lead to iatrogenic harm from overtreatment.
Questions/purposes
The purpose of this study was to assess changing prevalence, mortality, treatment complications, and resource consumption associated with PE after TJA before and after the introduction of CTPA.
Methods
The Nationwide Inpatient Sample was used to identify 2,335,248 patients undergoing TJA from 1993 to 1998 before the introduction of CTPA and 6,321,671 patients from 1999 to 2008 after the introduction of CTPA. Bivariate and multivariate regression analysis was performed to compare changing prevalence of PE, mortality, potential treatment complications of anticoagulation, length of stay, and total charges before and after the introduction of CTPA in patients with PE.
Results
In-hospital diagnosis of PE after TJA increased (p < 0.001) from an average of 0.27% to 0.37% after the introduction of CTPA. All-cause mortality in patients with a diagnosis of PE decreased (p < 0.001) from 11.5% to 4.6% (odds ratio, 2.3; 95% confidence interval, 2.1–2.6) after the introduction of CTPA. Overall, PE was associated with increased (p < 0.001) risks for hematoma/seroma, postoperative infection, gastrointestinal bleed, and drug-related thrombocytopenia, although the prevalence of these complications has decreased after 1998 (p < 0.001). Length of stay doubled for patients with PE (both before and after CTPA) and total charges increased over 69% in both study periods for these patients.
Conclusions
Adoption of CTPA appears to be associated with an increase in the diagnosis of PE after TJA and an associated decrease in case-fatality. Although CTPA may improve our ability to diagnose PE and possibly reduce mortality, the observed decrease in case-fatality could also be explained by the overdiagnosis of clinically unimportant emboli. The diagnosis of PE was strongly associated with potential iatrogenic harm from anticoagulation and increased length of stay and hospital charges in this study, emphasizing the importance of further investigation to define the role of CTPA in the diagnosis and treatment of PE after TJA.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Introduction
Venous thromboembolism (VTE) after hip and knee total joint arthroplasty (TJA) can result in consequences that range from deep vein thrombosis (DVT) and postphlebitis syndromes to saddle pulmonary embolus (PE) and death. The incidence of DVT when no prophylaxis is given ranges from 40% to 84% in patients undergoing TKA and 39% to 74% in patients undergoing THA [12, 30]. On the other hand, fatal PE associated with TJA is less common, ranging in incidence from 0.19% to 3.4% [19, 20, 30]. The goal of VTE prevention, detection, and treatment is to minimize the associated morbidity and mortality. Clinicians depend on imaging studies for diagnosis, because the history and physical examination are neither sensitive nor specific tools for making this diagnosis.
Multidetector row CT pulmonary angiography (CTPA) was introduced in 1998 as a highly sensitive test for diagnosing PE, replacing ventilation-perfusion scans. By 2006, several institutions reported a seven- to 13-fold increase in the use of CTPA [1, 9, 28, 34]. CTPA is now widely used by clinicians and considered the gold standard for diagnosing PE.
Because of the sensitivity of CTPA, small emboli may be detected that may not have any clinical significance. There is published literature suggesting that the introduction of CTPA may be associated with overdiagnosis, defined as the increase in detection of an abnormality that will never cause harm or death [29, 32]. Overdiagnosis of PE may explain the nationwide increase in the incidence of PE since the introduction of CTPA in 1998 [23]. Although clinically insignificant PEs are not harmful, substantial morbidity can be associated with the treatment of such emboli. Anticoagulation therapy can carry significant risks that include bleeding, wound complications, thrombocytopenic reaction, and death.
To our knowledge, the introduction of CTPA in the diagnosis of PE in patients undergoing primary TJA and the consequences of treatment have not been investigated. The purpose of this study was to assess the changing prevalence of PE, mortality, complications, and resource consumption associated with the treatment of PE after TJA both before and after the introduction of CTPA using a large, nationally representative database.
Patients and Methods
The Nationwide Inpatient Sample (NIS) was used to identify 8,656,919 patients who underwent primary THA and TKA in the United States between January 1, 1993, and December 31, 2008. This timeframe encompasses the 5 years before the introduction of CTPA (1993–1998) and the available years of data after its introduction (1998–2008) [32]. The NIS is a stratified, statistically valid survey of hospitals conducted by the federal Healthcare Cost and Utilization Project [15]. Hospitals are randomly selected to achieve an approximately 20% sample of the universe of hospitals. Sampling weights are provided to produce the national estimates. All discharge records from each of the selected hospitals are collected and form part of the NIS file for a given year. Because of the large size of the database, the NIS is particularly well suited for epidemiological studies related to specific procedures or diseases in the national population [3, 4].
The International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) procedure codes were used to identify procedures (codes 81.51 and 81.54), the diagnosis of PE (415.11 and 415.9), and potential in-hospital complications of anticoagulation for PE if these ICD-9 codes appeared in any of the 14 secondary diagnosis fields. Potential complications examined included gastrointestinal tract hemorrhage, coded as published by Witt et al. [33], drug-induced thrombocytopenia (code 287.4), hematoma/seroma (code 998.1X), and postoperative infection (code 998.5) [14, 24]. We also determined length of stay and total charges adjusted for inflation to 2008 US dollars to quantify the economic impact of the complications.
Of the total sample of 8,656,919 procedures, 35% were THAs and 65% were TKAs. Mean patient age was 67 years (range, 30–113 years), 61.5% were females, and the overall incidence of PE for the entire study period was 0.35%.
All statistical analyses were performed using SPSS Version 20 for Windows (IBM Corp, Armonk, NY, USA). Descriptive statistics were obtained for all variables used within the study. Missing values were excluded for the purpose of this analysis. Independent sample t-tests and chi-square analysis were used for bivariate comparisons. Multivariate modeling with logarithmic transformation was used to examine the risk-adjusted association between patients with and without PE. Each model was adjusted for the confounders of age, sex, type of procedure, and baseline comorbidities using the Deyo index [8]. Odds ratios were calculated with their respective 95% confidence intervals. For all comparisons and regressions, statistical significance was assigned at the p < 0.01 level given our large sample size with the increased probability of false-positive results (type I error).
Results
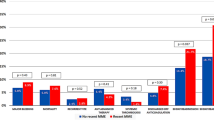
The prevalence of PE after TJA did not significantly change during the initial 5-year period before CTPA, but increased (p < 0.001) by 40% after CTPA was introduced, rising from an average of 0.27% in the first period to 0.37% in the second period (Fig. 1). All-cause mortality was higher (p < 0.001) in patients with the diagnosis of PE versus those without regardless of the study period (Table 1). However, mortality for patients with a diagnosis of PE decreased (p < 0.001) substantially from 11.5% before the introduction of CTPA to 4.6% after 1998. In our regression model, which controlled for age, sex, type of procedure, and baseline comorbidities, the odds ratio of mortality with a PE decreased after the introduction of CTPA from 38 to 28.
The diagnosis of PE was associated with increased (p < 0.001) risks for hematoma/seroma, postoperative infection, gastrointestinal bleed, and drug-related thrombocytopenia regardless of the study period (Table 1). In general, the reported prevalence of these complications (except drug-related thrombocytopenia) significantly decreased (p < 0.001) after the introduction of CTPA (Table 2).
The diagnosis of PE carried a significant increase (p < 0.001) in hospital length of stay (8.5 days versus 3.9 days) in the post-CTPA group. Length of stay after TJA progressively trended down during the study period, averaging 5.7 days pre- and 4 days post-CTPA. In the first period, patients with PE had an increased length of stay versus patients without PE of 12 versus 5.7 days (110% increase; p < 0.001). In the second period, the difference was 8.6 versus 3.9 days (120% increase; p < 0.001). Patients with PE had increased total charges (USD 71,657 versus USD 42,254; p < 0.001) in the post-CTPA group. This increase (69%) is similar to the 71% increase associated with a PE diagnosis before CTPA (USD 34,416 versus USD 58,977).
Discussion
VTE prophylaxis, diagnosis, and treatment modalities remain a concern as the rate of TJA continues to increase. Reported rates of symptomatic and asymptomatic DVT after TKA are approximately 40% to 50% when no prophylaxis is given and 16% to 27% when VTE prophylaxis is prescribed [10, 13, 18]. The rate of symptomatic VTE in patients undergoing TKA receiving prophylaxis is 1.3% to 2.3% [10, 11, 27]. The rate of PE in this population is generally reported with an incidence of less than 0.8% [6, 31], although there has been inconsistency regarding the incidence of VTE in association with TJA over the last 10 to 15 years [2, 16]. The purpose of this study was to analyze similar epidemiologic data as it pertains to patients undergoing primary TJA, specifically to assess the changing prevalence of PE along with the mortality, complications, and resource consumption associated with the treatment of PE after TJA both before and after the introduction of CTPA using a large, nationally representative database.
Many of the limitations of our study are inherent in the analysis of large administrative databases such as the NIS. Incomplete data collection, uncertain compliance and accuracy of coding related to diagnosis and procedures performed, and lack of detailed clinical information are all concerns. White et al. [31] have reported on the limitations of using the NIS for reporting PE (current versus historic embolism) and it is likely that this is reflected in our data. Also, with the current data, it is not possible to assess which PEs are clinically significant and which are not. Certain events such as postoperative hematoma are also open to a certain amount of subjective interpretation and potential for observer bias. Finally, given the nature of the data, direct mortality attributable to PE cannot be confirmed and we can merely report co-occurrence. These recognized limitations are inherent to all studies using this database design and could potentially be improved through prospective data collection. Despite these limitations, the large number of patients with PE included in our analysis provides unique and previously unavailable insight into the impact of this comorbidity and its treatment after primary joint arthroplasty throughout the United States.
The selection of 1998 as the time for introduction of CTPA has been used before [32], although there was not an universal uniform adoption of this test across the United States at the same time and we acknowledge this as a limitation of this study. However, a more progressive and gradual transition from the V/Q scan to CTPA during the initial years after 1998 may mean that the significant differences that we found are in fact underrepresented and that the difference in case-fatality rate is underestimated.
Since the introduction of CTPA in 1998, there has been an overall increase in the reported prevalence of PE nationwide [23]. This increase in the diagnosis of PE is concerning and may suggest that the widespread adoption of CTPA at the time of this observed increase may represent a phenomenon of overdiagnosis. This term can be defined as the increased detection of an abnormality that will never cause harm or death [29, 32]. Clinically insignificant emboli are more easily detected with CTPA, leading to more positive test results and thus a higher overall detection of PE. The present study demonstrates an average increase in PE diagnosis after TJA from 0.27% before the introduction of CTPA to 0.37% after 1998. This correlates with findings of an overall increase in the frequency of detection of PE in the general population [32]. A closer look at the epidemiology encompassing the recent increase in the diagnosis of PE suggests that associated mortality from PE has changed little and case-fatality has actually decreased [9]. Similarly, we also found that mortality associated with the diagnosis of PE decreased substantially from 11.5% to 4.6% after introduction of CTPA. Overdiagnosis may explain both the increased prevalence and decreased case-fatality observed. A decrease in case-fatality (the number of death/people diagnosed) may be attributable to inflation of the denominator as a result of an increased detection of clinically insignificant emboli. In this scenario, patients do not benefit from the increased sensitivity of CTPA, which detects more nonfatal PEs without reducing the number of fatal PEs, leaving patients still subject to potential harm from treatment. However, an alternative explanation exists. A decreased case-fatality rate could also be explained by the earlier diagnosis and improved sensitivity of PE detection afforded by the use of CTPA, which in turn reduces the number of fatal PEs. If the more sensitive CTPA detects more PEs and patients benefit from treatment, then CTPA becomes an effective test that may lead to fewer deaths. Whether patients benefit from the more sensitive test or are subject to potential iatrogenic harm without a death reduction benefit remains a question that cannot be answered by this study.
Our study does clearly demonstrate that the diagnosis of PE is associated with increased risks for morbidity and mortality. Although PE can lead to complications and death as a result of the disease process itself, the treatment of patients diagnosed with PE is not trivial and carries iatrogenic risks. The diagnosis of PE generally requires immediate anticoagulation. Complications associated with anticoagulation in the TJA population include bleeding, postoperative infection, a higher rate of blood product transfusion, and drug thrombocytopenia. Surgical site bleeding increases the risk of neurologic damage, infection, reoperation, and delays recovery [5, 7, 14, 17, 25, 26]. Neviaser et al. found that postoperative therapeutic enoxaparin treatment had a 10% rate of major and a 27% rate of minor bleeding complications in TJA patients [21]. Our study demonstrates that the diagnosis of PE was associated with increased risks of hematoma/seroma, postoperative infection, gastrointestinal bleed, and thrombocytopenia compared with patients who did not have this diagnosis. When comparing these treatment-related complications before and after the introduction of CTPA, the overall prevalence has decreased in patients with PE. To our knowledge, this finding has not been previously reported in patients undergoing primary TJA.
The diagnosis of PE also carried a significant increase in hospital length of stay. Similar differences of 5 days on average have been published in similar healthcare databases for patients undergoing primary TJA with postoperative VTE [22]. In our study, length of stay decreased overall from an average of 5.7 days in 1993 to 4 days in 2008. This decrease is likely related to improved postoperative pathways and more aggressive attempts to reduce length of inpatient stay. Patients diagnosed with PE had double the length of stay compared with those without a PE, a pattern that did not change after the introduction of CTPA. The increase in total charges associated with PE diagnosis in both time periods was also similar, suggesting the relative cost associated with PE diagnosis on a case-by-case basis has not dramatically changed.
There appears to be a correlation with the introduction of CTPA in 1998 and an increased diagnosis of PE after primary TJA. Case-fatality decreased in the observed time period, the explanation for which remains unknown; although the introduction of CTPA may have led to improvement in treatments and fewer deaths, there is concern that the increased sensitivity of CTPA has led to overdiagnosis of patients with nonfatal disease who do not benefit from intervention. Our data show that the diagnosis of PE is associated with potential iatrogenic harm from anticoagulation in addition to increasing length of stay and monetary cost. Further study is needed to determine the clinical significance of PE detected by CTPA and determine appropriate treatment algorithms that balance the risk of death versus the risk of iatrogenic harm from treatment. With the increased sensitivity offered by CTPA for diagnosing the size and anatomic location of PE, and given the association of anticoagulation therapy with postoperative complications, research-based guidelines are warranted to help determine a therapeutic or observational approach to emboli which may be clinically or physiologically insignificant.
References
Auer RC, Schulman AR, Tuorto S, Gonen M, Gonsalves J, Schwartz L, Ginsberg MS, Fong Y. Use of helical CT is associated with an increased incidence of postoperative pulmonary emboli in cancer patients with no change in the number of fatal pulmonary emboli. J Am Coll Surg. 2009;208:871–878; discussion 878–880.
Bjornara BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. 2006;88:386–391.
Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468:45–51.
Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128–133.
Butt AJ, McCarthy T, Kelly IP, Glynn T, McCoy G. Sciatic nerve palsy secondary to postoperative haematoma in primary total hip replacement. J Bone Joint Surg Br. 2005;87:1465–1467.
Colwell CW, Berkowitz SD, Lieberman JR, Comp PC, Ginsberg JS, Paiement G, McElhattan J, Roth AW, Francis CW, EXULT B Study Group. Oral direct thrombin inhibitor ximelagatran compared with warfarin for the prevention of venous thromboembolism after total knee arthroplasty. J Bone Joint Surg Am. 2005;87:2169–2177
Decousus H, Leizorovicz A, Parent F, Page Y, Tardy B, Girard P, Laporte S, Faivre R, Charbonnier B, Barral FG, Huet Y, Simonneau G. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prevention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338:409–415.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619.
Donohoo JH, Mayo-Smith WW, Pezzullo JA, Egglin TK. Utilization patterns and diagnostic yield of 3421 consecutive multidetector row computed tomography pulmonary angiograms in a busy emergency department. J Comput Assist Tomogr. 2008;32:421–425.
Douketis JD, Eikelboom JW, Quinlan DJ, Willan AR, Crowther MA. Short-duration prophylaxis against venous thromboembolism after total hip or knee replacement: a meta-analysis of prospective studies investigating symptomatic outcomes. Arch Intern Med. 2002;162:1465–1471.
Francis CW, Berkowitz SD, Comp PC, Lieberman JR, Ginsberg JS, Paiement G, Peters GR, Roth AW, McElhattan J, Colwell CW Jr, EXULT A Study Group. Comparison of ximelagatran with warfarin for the prevention of venous thromboembolism after total knee replacement. N Engl J Med. 2003;349:1703–1712.
Freedman KB, Brookenthal KR, Fitzgerald RH Jr, Williams S, Lonner JH. A meta-analysis of thromboembolic prophylaxis following elective total hip arthroplasty. J Bone Joint Surg Am. 2000;82:929–938.
Fujita S, Hirota S, Oda T, Kato Y, Tsukamoto Y, Fuji T. Deep venous thrombosis after total hip or total knee arthroplasty in patients in Japan. Clin Orthop Relat Res. 2000;375:168–174.
Galat DD, McGovern SC, Hanssen AD, Larson DR, Harrington JR, Clarke HD. Early return to surgery for evacuation of a postoperative hematoma after primary total knee arthroplasty. J Bone Joint Surg Am. 2008;90:2331–2336.
HCUP. Introduction to the HCUP Nationwide Inpatient Sample (NIS) 2005. Rockville, MD, USA: Agency for Healthcare Quality and Research (AHRQ); 2007.
Howie C, Hughes H, Watts AC. Venous thromboembolism associated with hip and knee replacement over a ten-year period: a population-based study. J Bone Joint Surg Br. 2005;87:1675–1680.
Keays AC, Mason M, Keays SL, Newcombe PA. The effect of anticoagulation on the restoration of range of motion after total knee arthroplasty: enoxaparin versus aspirin. J Arthroplasty. 2003;18:180–185.
Kim YH, Kim JS. Incidence and natural history of deep-vein thrombosis after total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 2002;84:566–570.
Kim YH, Oh SH, Kim JS. Incidence and natural history of deep-vein thrombosis after total hip arthroplasty. A prospective and randomised clinical study. J Bone Joint Surg Br. 2003;85:661–665.
Lawton RL, Morrey BF, Narr BJ. Validity of index of suspicion for pulmonary embolism after hip arthroplasty. Clin Orthop Relat Res. 2003;415:180–192.
Neviaser AS, Chang C, Lyman S, Della Valle AG, Haas SB. High incidence of complications from enoxaparin treatment after arthroplasty. Clin Orthop Relat Res. 2010;468:115–119.
Oster G, Ollendorf DA, Vera-Llonch M, Hagiwara M, Berger A, Edelsberg J. Economic consequences of venous thromboembolism following major orthopedic surgery. Ann Pharmacother. 2004;38:377–382.
Park B, Messina L, Dargon P, Huang W, Ciocca R, Anderson FA. Recent trends in clinical outcomes and resource utilization for pulmonary embolism in the United States: findings from the nationwide inpatient sample. Chest. 2009;136:983–990.
Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does ‘excessive’ anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22:24–28.
Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89:33–38.
Sanchez-Ballester J, Smith M, Hassan K, Kershaw S, Elsworth CS, Jacobs L. Wound infection in the management of hip fractures: a comparison between low-molecular weight heparin and mechanical prophylaxis. Acta Orthop Belg. 2005;71:55–59.
Warwick D, Friedman RJ, Agnelli G, Gil-Garay E, Johnson K, FitzGerald G, Turibio FM. Insufficient duration of venous thromboembolism prophylaxis after total hip or knee replacement when compared with the time course of thromboembolic events: findings from the Global Orthopaedic Registry. J Bone Joint Surg Br. 2007;89:799–807.
Weir ID, Drescher F, Cousin D, Fraser ET, Lee R, Berman L, Strauss E, Wang Y, Fine JM. Trends in use and yield of chest computed tomography with angiography for diagnosis of pulmonary embolism in a Connecticut hospital emergency department. Conn Med. 2010;74:5–9.
Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–613.
Westrich GH, Haas SB, Mosca P, Peterson M. Meta-analysis of thromboembolic prophylaxis after total knee arthroplasty. J Bone Joint Surg Br. 2000;82:795–800.
White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 1998;158:1525–1531.
Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171:831–837.
Witt DM, Delate T, Clark NP, Martell C, Tran T, Crowther MA, Garcia DA, Ageno W, Hylek EM, Warfarin Associated Research Projects and other EnDeavors (WARPED) Consortium. Outcomes and predictors of very stable INR control during chronic anticoagulation therapy. Blood. 2009;114:952–956.
Wittram C, Meehan MJ, Halpern EF, Shepard JA, McLoud TC, Thrall JH. Trends in thoracic radiology over a decade at a large academic medical center. J Thorac Imaging. 2004;19:164–170.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
About this article
Cite this article
D’Apuzzo, M.R., Keller, T.C., Novicoff, W.M. et al. CT Pulmonary Angiography After Total Joint Arthroplasty: Overdiagnosis and Iatrogenic Harm?. Clin Orthop Relat Res 471, 2737–2742 (2013). https://doi.org/10.1007/s11999-013-3041-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-013-3041-4