Abstract
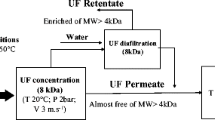
Continuous processing in the production of peptides is an area of increased interest. In this study, an enzymatic membrane reactor (EMR) was developed whereby whey protein isolate was used as a substrate to prepare DPP-IV inhibitory and radical scavenging peptides via enzymatic hydrolysis. Two separate enzymes were tested: Corolase 2TS and Protamex in conventional batch processes and the EMR. Neither enzyme was considered effective at producing peptides with radical scavenging activity when measured using a DPPH assay. However, both enzymes were capable of producing DPP-IV inhibitory peptides. Corolase and Protamex both produced similar DPP-IV inhibition levels upon completion of batch experiments. In the EMR process, permeate in the Protamex run showed 33.7% lower IC50 value compared to the continuous Corolase run. Protamex was a better enzyme at producing the DPP-IV inhibitory effect. The continuous (EMR) production method showed an increased productivity over batch for both enzymes.





Similar content being viewed by others
References
Aart, V., Catharina M., Zeeland-Wolbers V., Maria L., Gilst V., Hendrikus W., Nelissen B. and Maria J. (2009). "Egg protein hydrolysates." Patent, WO 128713: 2009.
Adler-Nissen, J. (1986). Enzymic hydrolysis of food proteins. New York: Elsevier Applied Science Publishers.
Brandelli, A., Daroit, D. J., & Corrêa, A. P. F. (2015). Whey as a source of peptides with remarkable biological activities. Food Research International, 73, 149–161.
Cabrera-Padilla, R. Y., Pinto, G. A., Giordano, R. L., & Giordano, R. C. (2009). A new conception of enzymatic membrane reactor for the production of whey hydrolysates with low contents of phenylalanine. Process Biochemistry, 44(3), 269–276.
Cheison, S. C., Wang, Z., & Xu, S.-Y. (2006). Hydrolysis of whey protein isolate in a tangential flow filter membrane reactor: I. Characterisation of permeate flux and product recovery by multivariate data analysis. Journal of Membrane Science, 283(1), 45–56.
Corrêa, A. P. F., Daroit, D. J., Fontoura, R., Meira, S. M. M., Segalin, J., & Brandelli, A. (2014). Hydrolysates of sheep cheese whey as a source of bioactive peptides with antioxidant and angiotensin-converting enzyme inhibitory activities. Peptides, 61, 48–55.
Eisele, T., Stressler, T., Kranz, B., & Fischer, L. (2013). Bioactive peptides generated in an enzyme membrane reactor using Bacillus lentus alkaline peptidase. European Food Research and Technology, 236(3), 483–490.
Erdős, B., Grachten, M., Czermak, P., & Kovács, Z. (2018). Artificial neural network-assisted spectrophotometric method for monitoring Fructo-oligosaccharides production. Food and Bioprocess Technology, 11(2), 305–313.
Ha, G. E., Chang, O. K., Jo, S. M., Han, G. S., Park, B. Y., Ham, J. S., & Jeong, S. G. (2015). Identification of antihypertensive peptides derived from low molecular weight casein hydrolysates generated during fermentation by Bifidobacterium longum KACC 91563. Korean Journal for Food Science of Animal Resources, 35(6), 738–747.
Haug, A., Hostmark, A. T., & Harstad, O. M. (2007). Bovine milk in human nutrition – A review. Lipids in Health and Disease, 6(1), 25.
Hernández-Ledesma, B., Recio, I., & Amigo, L. (2008). β-Lactoglobulin as source of bioactive peptides. Amino Acids, 35(2), 257–265.
ISO F. (2009). Feed products–general guidelines for the determination of nitrogen by the Kjeldahl method. International Organization for Standardiza-tion, Geneva, Switzerland, 1871, 2009.
Jakovetić, S., Luković, N., Jugović, B., Gvozdenović, M., Grbavčić, S., Jovanović, J., & Knežević-Jugović, Z. (2015). Production of antioxidant egg white hydrolysates in a continuous stirred tank enzyme reactor coupled with membrane separation unit. Food and Bioprocess Technology, 8(2), 287–300.
Janson, J.-C. (2012). Protein purification: Principles, high resolution methods, and applications, John Wiley & Sons.
Jemil, I., Jridi, M., Nasri, R., Ktari, N., Salem, R. B. S.-B., Mehiri, M., Hajji, M., & Nasri, M. (2014). Functional, antioxidant and antibacterial properties of protein hydrolysates prepared from fish meat fermented by Bacillus subtilis A26. Process Biochemistry, 49(6), 963–972.
Juillerat-Jeanneret, L. (2013). Dipeptidyl peptidase IV and its inhibitors: Therapeutics for type 2 diabetes and what else? Journal of Medicinal Chemistry, 57(6), 2197–2212.
Kamau, S. M., & Lu, R.-R. (2011). The effect of enzymes and hydrolysis conditions on degree of hydrolysis and DPPH radical scavenging activity of whey protein hydrolysates. Current Research in Dairy Sciences, 3, 25–35.
Lacroix, I. M., Meng, G., Cheung, I. W., & Li-Chan, E. C. (2016). Do whey protein-derived peptides have dual dipeptidyl-peptidase IV and angiotensin I-converting enzyme inhibitory activities? Journal of Functional Foods, 21, 87–96.
Le Maux, S., Nongonierma, A. B., Barre, C., & FitzGerald, R. J. (2016). Enzymatic generation of whey protein hydrolysates under pH-controlled and non pH-controlled conditions: Impact on physicochemical and bioactive properties. Food Chemistry, 199, 246–251.
Le Maux, S., Nongonierma, A. B., & FitzGerald, R. J. (2015a). Improved short peptide identification using HILIC–MS/MS: Retention time prediction model based on the impact of amino acid position in the peptide sequence. Food Chemistry, 173, 847–854.
Le Maux, S., Nongonierma, A. B., Murray, B., Kelly, P. M., & FitzGerald, R. J. (2015b). Identification of short peptide sequences in the nanofiltration permeate of a bioactive whey protein hydrolysate. Food Research International, 77, 534–539.
Manders, R. J., Hansen, D., Zorenc, A. H., Dendale, P., Kloek, J., Saris, W. H., & van Loon, L. J. (2014). Protein co-ingestion strongly increases postprandial insulin secretion in type 2 diabetes patients. Journal of Medicinal Food, 17(7), 758–763.
Mateo, C., Palomo, J. M., Fernandez-Lorente, G., Guisan, J. M., & Fernandez-Lafuente, R. (2007). Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme and Microbial Technology, 40(6), 1451–1463.
Méric, E., Lemieux, S., Turgeon, S. L., & Bazinet, L. (2014). Insulin and glucose responses after ingestion of different loads and forms of vegetable or animal proteins in protein enriched fruit beverages. Journal of Functional Foods, 10, 95–103.
Nath, A., Verasztó, B., Basak, S., Koris, A., Kovács, Z., & Vatai, G. (2016). Synthesis of lactose-derived nutraceuticals from dairy waste whey—A review. Food and Bioprocess Technology, 9(1), 16–48.
Noble, R. D. and S. A. Stern (1995). Membrane separations technology: Principles and applications, Elsevier.
Nongonierma, A. B., & FitzGerald, R. J. (2013a). Dipeptidyl peptidase IV inhibitory and antioxidative properties of milk protein-derived dipeptides and hydrolysates. Peptides, 39, 157–163.
Nongonierma, A. B., & FitzGerald, R. J. (2013b). Inhibition of dipeptidyl peptidase IV (DPP-IV) by proline containing casein-derived peptides. Journal of Functional Foods, 5(4), 1909–1917.
Nongonierma, A. B., & FitzGerald, R. J. (2013c). Inhibition of dipeptidyl peptidase IV (DPP-IV) by tryptophan containing dipeptides. Food & Function, 4(12), 1843–1849.
Nongonierma, A. B., & FitzGerald, R. J. (2016). Prospects for the management of type 2 diabetes using food protein-derived peptides with dipeptidyl peptidase IV (DPP-IV) inhibitory activity. Current Opinion in Food Science, 8, 19–24.
Peng, X., Kong, B., Xia, X., & Liu, Q. (2010). Reducing and radical-scavenging activities of whey protein hydrolysates prepared with Alcalase. International Dairy Journal, 20(5), 360–365.
Peng, X., Xiong, Y. L., & Kong, B. (2009). Antioxidant activity of peptide fractions from whey protein hydrolysates as measured by electron spin resonance. Food Chemistry, 113(1), 196–201.
Rodrigues, R. C., Ortiz, C., Berenguer-Murcia, Á., Torres, R., & Fernández-Lafuente, R. (2013). Modifying enzyme activity and selectivity by immobilization. Chemical Society Reviews, 42(15), 6290–6307.
Seader, J. D. and E. J. Henley (2011). "Separation process principles." John Wiley and Sons, Inc.
Solieri, L., Rutella, G. S., & Tagliazucchi, D. (2015). Impact of non-starter lactobacilli on release of peptides with angiotensin-converting enzyme inhibitory and antioxidant activities during bovine milk fermentation. Food Microbiology, 51, 108–116.
Spellman, D., McEvoy, E., O’cuinn, G., & FitzGerald, R. (2003). Proteinase and exopeptidase hydrolysis of whey protein: Comparison of the TNBS, OPA and pH stat methods for quantification of degree of hydrolysis. International Dairy Journal, 13(6), 447–453.
Tong, L. M., Sasaki, S., McClements, D. J., & Decker, E. A. (2000). Mechanisms of the antioxidant activity of a high molecular weight fraction of whey. Journal of Agricultural and Food Chemistry, 48(5), 1473–1478.
Van Reis, R., & Zydney, A. (2001). Membrane separations in biotechnology. Current Opinion in Biotechnology, 12(2), 208–211.
Zambrowicz, A., Polanowski, T. A., Lubec, G., & Trziszka, T. (2013). Manufacturing of peptides exhibiting biological activity. Amino Acids, 44(2), 315–320.
Zhu, C.-F., Li, G.-Z., Peng, H.-B., Zhang, F., Chen, Y., & Li, Y. (2010). Treatment with marine collagen peptides modulates glucose and lipid metabolism in Chinese patients with type 2 diabetes mellitus. Applied Physiology Nutrition and Metabolism, 35(6), 797–804.
Acknowledgements
Enterprise Ireland is acknowledged for financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
O’Halloran, J., O’Sullivan, M. & Casey, E. Production of Whey-Derived DPP-IV Inhibitory Peptides Using an Enzymatic Membrane Reactor. Food Bioprocess Technol 12, 799–808 (2019). https://doi.org/10.1007/s11947-019-02253-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-019-02253-7