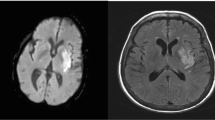
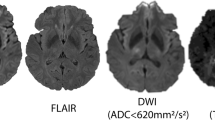
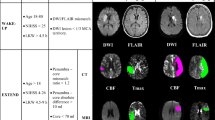
Abstract
Purpose of the review
Advanced imaging is now used to identify acute ischemic stroke (AIS) patients for treatment with tissue plasminogen activator or mechanical thrombectomy, who are either out of the traditional treatment window or have an unknown last known well time. Current guidelines recommend adapting the complex inclusion criteria used in the clinical trials to provide optimal individual patient decisions in clinical practice. This review aims to facilitate such integration by organizing the available data in a practical clinical context.
Recent findings
We review two clinical vignettes of advanced imaging for the treatment of AIS, the rationale for using the imaging criteria with correlations from the gold-standard quantitative oxygen-15 positron emission tomography imaging to understand cerebral ischemia and magnetic resonance imaging, and its limitations.
Summary
Advanced stroke imaging is required to treat patients with AIS in the extended time window. Understanding the rationale, differences in the individual study criteria and limitations of imaging can help the treating physician in incorporating advanced stroke imaging-based clinical trials in clinical practice.


Similar content being viewed by others
References and Recommended Reading
Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21. A randomized clinical trial of 206 patients that supports the use of mechanical thrombectomy in 6–24 hours from last known well time in patients with a large vessel occlusion and clinical imaging mismatch.
Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–18. A randomized clinical trial of 182 patients that showed mechanical thrombectomy in 6–16 hours from last known well time has better functional outcomes compared to standard medical therapy.
Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–31.
Ma H, Campbell BCV, Parsons MW, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med 2019;380:1795–803. A randomized clinical trial of 225 patients that showed treatment with intravenous alteplase between 4.5 to 9 hours of stroke onset resulted in excellent functional outcome compared to placebo.
Schwamm LH, Wu O, Song SS, et al. Intravenous thrombolysis in unwitnessed stroke onset: MR WITNESS trial results. Ann Neurol 2018;83:980–93. A phase II clinical trial of 80 patients that showed treatment with intravenous alteplase was safe in patients with unwitnessed stroke and MRI findings of quantitative DWI-FLAIR mismatch.
Thomalla G, Simonsen CZ, Boutitie F, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med 2018;379:611–22. A randomized clinical trial of 503 patients that showed treatment with intravenous alteplase was safe and effective with better functional outcomes in patients with unknown time of stroke onset and MRI findings of DWI-FLAIR mismatch
Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–418.
Leigh R, Knutsson L, Zhou J, van Zijl PC. Imaging the physiological evolution of the ischemic penumbra in acute ischemic stroke. J Cereb Blood Flow Metab 2017:271678X17700913. A review article on hemodynamic changes in acute ischemic stroke with focus on PET studies and perfusion imaging.
Bandera E, Botteri M, Minelli C, et al. Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke: a systematic review. Stroke. 2006;37:1334–9.
Markus HS. Cerebral perfusion and stroke. J Neurol Neurosurg Psychiatry. 2004;75:353–61.
Zaro-Weber O, Fleischer H, Reiblich L, et al. Penumbra detection in acute stroke with perfusion magnetic resonance imaging: validation with (15) O-positron emission tomography. Ann Neurol 2019;85:875–886. A PET study that validates the use of Tmax threshold (>5.6 seconds) in perfusion imaging to detect ischemic penumbra.
Lassen NA. Normal average value of cerebral blood flow in younger adults is 50 ml/100 g/min. J Cereb Blood Flow Metab. 1985;5:347–9.
Goyal M, Menon BK, Derdeyn CP. Perfusion imaging in acute ischemic stroke: let us improve the science before changing clinical practice. Radiology. 2013;266:16–21.
Vagal A, Wintermark M, Nael K, et al. Automated CT perfusion imaging for acute ischemic stroke: Pearls and pitfalls for real-world use. Neurology. 2019;93:888–98.
Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309.
Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–17.
Kakuda W, Lansberg MG, Thijs VN, et al. Optimal definition for PWI/DWI mismatch in acute ischemic stroke patients. J Cereb Blood Flow Metab. 2008;28:887–91.
Mlynash M, Lansberg MG, De Silva DA, et al. Refining the definition of the malignant profile: insights from the DEFUSE-EPITHET pooled data set. Stroke. 2011;42:1270–5.
Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11:860–7.
Amukotuwa S, Straka M, Aksoy D, et al. Cerebral blood flow predicts the infarct core: new insights from contemporaneous diffusion and perfusion imaging. Stroke. 2019;50:2783–9.
Mokin M, Levy EI, Saver JL, et al. Predictive value of RAPID assessed perfusion thresholds on final infarct volume in SWIFT PRIME (Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment). Stroke. 2017;48:932–8.
Lin L, Bivard A, Krishnamurthy V, et al. Whole-brain CT perfusion to quantify acute ischemic penumbra and core. Radiology. 2016;279:876–87.
Cereda CW, Christensen S, Campbell BCV, et al. A benchmarking tool to evaluate computer tomography perfusion infarct core predictions against a DWI standard. J Cereb Blood Flow Metab 2016;36:1780–1789. A CTP-MRI study that showed CBF thresholds to identify ischemic core.
Zaro-Weber O, Moeller-Hartmann W, Heiss WD, Sobesky J. Maps of time to maximum and time to peak for mismatch definition in clinical stroke studies validated with positron emission tomography. Stroke. 2010;41:2817–21.
Takasawa M, Jones PS, Guadagno JV, et al. How reliable is perfusion MR in acute stroke? Validation and determination of the penumbra threshold against quantitative PET. Stroke. 2008;39:870–7.
Guadagno JV, Warburton EA, Jones PS, et al. The diffusion-weighted lesion in acute stroke: heterogeneous patterns of flow/metabolism uncoupling as assessed by quantitative positron emission tomography. Cerebrovasc Dis. 2005;19:239–46.
Guadagno JV, Warburton EA, Jones PS, et al. How affected is oxygen metabolism in DWI lesions?: a combined acute stroke PET-MR study. Neurology. 2006;67:824–9.
Nagaraja N, Forder JR, Warach S, Merino JG. Reversible diffusion-weighted imaging lesions in acute ischemic stroke: a systematic review. Neurology. 2020;94:571–87.
Bivard A, Kleinig T, Miteff F, et al. Ischemic core thresholds change with time to reperfusion: a case control study. Ann Neurol. 2017;82:995–1003.
Rimmele DL, Thomalla G. Wake-up stroke: clinical characteristics, imaging findings, and treatment option - an update. Front Neurol. 2014;5:35.
Mackey J, Kleindorfer D, Sucharew H, et al. Population-based study of wake-up strokes. Neurology. 2011;76:1662–7.
Fink JN, Kumar S, Horkan C, et al. The stroke patient who woke up: clinical and radiological features, including diffusion and perfusion MRI. Stroke. 2002;33:988–93.
Thomalla G, Cheng B, Ebinger M, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4.5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol. 2011;10:978–86.
Thomalla G, Gerloff C. We are on the clock: MRI as a surrogate marker of lesion age in acute ischemic stroke. Stroke. 2010;41:197–8.
Ebinger M, Galinovic I, Rozanski M, et al. Fluid-attenuated inversion recovery evolution within 12 hours from stroke onset: a reliable tissue clock? Stroke. 2010;41:250–5.
Aoki J, Kimura K, Iguchi Y, et al. FLAIR can estimate the onset time in acute ischemic stroke patients. J Neurol Sci. 2010;293:39–44.
Song SS, Latour LL, Ritter CH, et al. A pragmatic approach using magnetic resonance imaging to treat ischemic strokes of unknown onset time in a thrombolytic trial. Stroke. 2012;43:2331–5.
Thomalla G, Rossbach P, Rosenkranz M, et al. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 hours or less. Ann Neurol. 2009;65:724–32.
Miller DJ, Simpson JR, Silver B. Safety of thrombolysis in acute ischemic stroke: a review of complications, risk factors, and newer technologies. Neurohospitalist. 2011;1:138–47.
Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nandakumar Nagaraja is a stroke outcome adjudicator for the Women’s Health Initiative study and receives small per-case stipend for this role and receives research/salary support from the NIH and McJunkin Foundation Alzheimer’s Disease Fund through 1Florida Alzheimer’s Disease Research Center. José G Merino receives salary support from the American Academy of Neurology for his role as Editor-in-Chief of Neurology. Enrique Leira receives salary support from NIH-NINDS. Steven Warach is a consultant for Genentech and chair of the independent data monitoring committee for the TIMELESS trial.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cerebrovascular Disorders
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nagaraja, N., Merino, J.G., Leira, E.C. et al. Advanced Imaging in the Era of Tissue-Based Treatment for Acute Ischemic Stroke—a Practical Review. Curr Treat Options Neurol 23, 32 (2021). https://doi.org/10.1007/s11940-021-00685-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s11940-021-00685-1