Abstract
Purpose of review
During the past 25 years, there has been an explosion of information regarding the occurrence of gastrointestinal dysfunction in Parkinson’s disease. In this review, the clinical features of gastrointestinal dysfunction in Parkinson’s disease will be described and information regarding the potential role of the enteric nervous system and the gut microbiome in the genesis of Parkinson’s disease will be addressed.
Recent findings
Recognition is growing regarding the role that gastroparesis and small intestinal dysfunction may play in Parkinson’s disease, especially with regard to erratic responses to anti-Parkinson medication. The presence of enteric nervous system involvement in Parkinson’s disease is now well established, but whether the enteric nervous system is the starting point for Parkinson’s disease pathology remains a source of debate. The potential role of the gut microbiome also is beginning to emerge.
Summary
Gastrointestinal dysfunction is a prominent nonmotor feature of Parkinson’s disease and dysfunction can be found along the entire length of the gastrointestinal tract. The enteric nervous system is clearly involved in Parkinson’s disease. Whether it is the initial source of pathology is still a source of controversy. There also is growing recognition of the role that the gut microbiome may play in Parkinson’s disease, but much more research is needed to fully assess this aspect of the disorder.
Similar content being viewed by others
Introduction
In writing his 1817 treatise, James Parkinson was clearly aware that gastrointestinal (GI) dysfunction was part of the clinical spectrum of what we now know as Parkinson’s disease (PD). He described dysphagia: “….so much are the actions of the muscles of the tongue, pharynx, &c. impeded by impaired action and perpetual agitation, that the food is with difficulty retained in the mouth until masticated; and then as difficultly swallowed.” [1•] He was aware of the tendency for individuals with PD to have increased saliva with drooling: “the saliva fails of being directed to the back part of the fauces, and hence is continually draining from the mouth.” [1•] He even had the perspicacity to not simply mention bowel dysfunction but to actually describe two distinct aspects of bowel dysfunction in PD in the forms of reduced bowel movement frequency and difficulty with the act of defecation itself: “the bowels, which had been all along torpid, now, in most cases, demand stimulating medicines of very considerable power: the expulsion of faeces from the rectum sometimes requiring mechanical aid.” [1•]
Curiously, following Dr. Parkinson’s publication, next to nothing was written about GI dysfunction in PD for almost 150 years. Eadie and Tyrer published a report in 1965 that described aspects of GI dysfunction in PD [2], but subsequently little more was written for another quarter century. That drought finally came to an end, and in the past 25 years, there has been a veritable flood of reporting and writing about not only the clinical features of GI dysfunction in PD but also their pathophysiological basis, and currently attention has focused on the possible role that changes in the gut may play in the genesis of the disease itself.
In light of this, it is both fitting and remarkable that James Parkinson himself, in that 1817 treatise, also speculated about the possibility that PD might have its roots within the GI system and spread from there to the central nervous system, when he wrote: “Although unable to trace the connection by which a disordered state of the stomach and bowels may induce a morbid action in a part of the medulla spinalis, yet taught by the instruction of Mr. Abernethy, little hesitation need be employed before we determine on the probability of such occurrence.” [1•]
In this review, the clinical features of GI dysfunction in PD will be discussed, the pathophysiological basis for these clinical features will be addressed, and the evolving topic of the gut microbiome and its potential role in the genesis of PD will broached.
GI features of PD
A resurgence of interest in the GI manifestations of PD was heralded by the report of Edwards and colleagues in 1991 delineating five GI features—excess saliva, dysphagia, nausea, decreased bowel movement frequency, and difficulty with the act of defecation itself—as occurring more frequently in PD patients than in comparably aged controls [3]. It was suggested that, beyond the obvious medication-induced nausea, impaired gastric emptying might also be a cause of nausea and other symptoms in PD [4]. Subsequent studies involving larger numbers and using validated questionnaires [5] confirmed these initial findings and more recent studies have documented the presence of these and some additional abnormalities of GI function in the setting of PD, which will be addressed in this review.
Excess saliva
The reported presence of excess saliva in the mouth of individuals with PD is 10–81% [6]. It is surprising to learn, then, that saliva production actually is decreased in PD, rather than increased [7]. The reason for the drooling is that individuals with PD no longer swallow as frequently or as efficiently as normal, which allows the saliva to accumulate in the mouth. The predilection for stooped posture and for the jaw to drop slightly, leaving the mouth open, then leads to the drooling. Initially, it may be sufficient to have the PD patient simply chew gum or suck on hard candy in social situations, which turns the impaired automatic swallowing into a more conscious maneuver. If more extended relief is needed, approaches that reduce saliva production are necessary. Systemic anticholinergic drugs that cross the blood-brain barrier are perhaps best avoided, but ones that do not, such as glycopyrrolate, may be useful. An alternative to systemic anticholinergics is the sublingual administration of a drop of atropine 1% ophthalmic solution once or twice a day [8]. If such simple measures are ineffective, botulinum toxin injections into the salivary glands typically produce improvement that lasts for 3–4 months [9].
Dysphagia
Subjective survey studies report the presence of dysphagia in 9–82% of individuals with PD, but objective studies, such as modified barium swallow studies (MBSS), demonstrate at least some abnormality in 75–97% of individuals [6, 10, 11]. A recent controlled, cross-sectional study of 119 PD patients, utilizing flexible endoscopic evaluation of swallowing (FEES), was able to demonstrate a completely normal examination in only 5% of PD patients [12]. Thus, clinically asymptomatic abnormalities of swallowing may be present in a significant number of persons with PD. Any phase of swallowing—oral, pharyngeal, or esophageal—may be affected. Rigidity and bradykinesia of the 30 pairs of striated muscles involved in the oral and pharyngeal phases of swallowing, along with dysmotility involving both the striated and smooth musculature of the esophagus, are likely responsible, although it also has been suggested that impaired pharyngeal sensation may play a role [13]. A variety of abnormalities of oropharyngeal and esophageal dysfunction have been identified, including abnormalities of tongue function resulting in abnormal bolus formation and transfer, pharyngeal dyscoordination, and esophageal dysmotility. It is important to remember that other processes, which may or may not occur more frequently in PD, may also be responsible for dysphagia in individuals with PD. Examples of this include the presence of anterior cervical osteophytes impinging on the esophagus, Zenker’s diverticulum, cricopharyngeal bar, achalasia, and even severe gastroesophageal reflux [14, 15].
Aspiration is an important risk for individuals with PD and dysphagia. Some studies suggest that over 50% of persons with PD will display at least some radiographic evidence of aspiration, although the aspiration may be clinically silent in a significant percentage of those affected [16, 17].
Treatment of dysphagia in PD depends upon the cause. If an MBSS identifies disordered oropharyngeal or upper esophageal swallowing indicative of PD, referral to a speech/swallowing therapist for training is appropriate. Whether adjustment of PD medication can be helpful is still a subject of debate [18,19,20]. If the MBSS demonstrates a specific abnormality, such as a Zenker’s diverticulum or cricopharyngeal bar, appropriate surgical or other therapy can be utilized [15]. If the MBSS does not demonstrate an abnormality that accounts for the dysphagia, more thorough visualization of the distal portion of the esophagus by esophagram should be considered.
Gastroparesis
Impaired or delayed gastric emptying (gastroparesis) is characterized not only by nausea, but also by reduced appetite, early satiety, bloating, vomiting, and weight loss [21]. Impaired antral contractions or failure of the pyloric sphincter to relax may be responsible. Gastroparesis already may be evident in early and untreated PD, but its presence and severity tends to become more prominent with disease progression [22,23,24]. Scintigraphic gastric emptying studies (solid, liquid, or both) have been the standard means by which to confirm the presence of gastroparesis. However, the recent availability of the wireless motility capsule testing system may prove to be a more advantageous method to obtain information about not only gastric emptying but also other aspects of GI motility [25, 26].
It is important to remember that, in addition to producing the GI symptoms mentioned above, gastroparesis can also be responsible for inconsistent responses to levodopa, including both delayed response and complete dose failure, since levodopa must reach the small intestine in order to be absorbed. Other processes, such as competition with protein for absorption in the small intestine, levodopa tablet sequestration in the epiglottic valleculae, Helicobacter pylori infection, and small intestinal bacterial overgrowth may also produce erratic responses to levodopa [27, 28]. Recently, impaired gastric emptying secondary to an enormous hiatal hernia also was reported to produce levodopa dose failure [29].
Treatment of gastroparesis in PD can be problematic. The standard method for treating gastroparesis in other situations, metoclopramide, cannot be used in PD patients because it crosses the blood-brain barrier and will block dopamine receptors within the central nervous system. Domperidone does not cross the blood-brain barrier but has never been approved for use in the USA. The motilin agonist, erythromycin, is not appropriate for extended use. Serotonin 5-HT4 agonists, which act by increasing acetylcholine release, have either been withdrawn from use (cisapride, tegaserod) or have never been approved for use in the USA (prucalopride, mosapride, renzapride). One small pilot study has reported the effectiveness of the histamine H2 receptor blocker, nizatidine, in improving gastroparesis in PD, but further confirmation is lacking [30]. Ghrelin agonists, such as relamorelin, appear to be potentially useful prokinetic agents, but are still experimental [31]. Several non-pharmacological treatment approaches are worth mentioning. If gastroparesis is due to failure of the pyloric sphincter to relax, which has been suggested to be the case in at least some individuals with PD, botulinum toxin injections into the pyloric sphincter may alleviate gastroparesis. The effectiveness of this approach has been described by several groups of investigators [32, 33]. Gastric pacemaker implantation has been utilized in individuals with severe, treatment-resistant gastroparesis caused by diabetes, but there is no published experience in PD [34].
Small intestinal dysfunction
The small intestine has received very little research attention in the setting of PD, probably as a consequence of its relative inaccessibility. However, small intestinal bacterial overgrowth (SIBO) has been reported to be present in 54% of PD patients in one recent study, with the presumption that impaired small intestinal motility may be responsible [35]. SIBO is characterized by increased bacterial density in the small intestine, including the presence of bacterial species originating in the colon. It may be characterized by flatulence and a sense of bloating [35]. If sufficiently severe, SIBO may cause malabsorption, which could conceivably explain the weight loss so frequently evident in PD.
In another interesting study, Fasano and colleagues reported that individuals with PD and SIBO were more likely to experience erratic responses to levodopa, such as increased “off” time, delayed responses, and dose failures, than were those without SIBO [27]. Moreover, antibiotic treatment of the SIBO resulted in improvement in these parameters, at least for a period of time.
In a more recent and larger study involving 103 individuals with PD, Tan and colleagues identified SIBO in 25.3% of the patients [36]. The presence of SIBO was not associated with more prominent GI symptoms, but its presence did predict increased motor dysfunction.
Delayed colon transit constipation
Slowed colon transit time has been documented in PD by numerous investigators, with transit times often about twice as long as in control individuals [37,38,39,40]. At least two studies have indicated that slowed colon transit is evident in approximately 80% of individuals with PD [37, 40] and the slowed transit presumably is responsible for less frequent bowel movements. As with dysphagia, objective impairment of colon transit is present in a greater percentage of PD patients than are subjective symptoms [40]. Recent experience suggests that PD constipation may differ from idiopathic constipation in some other ways. In a study evaluating the possible efficacy of the ghrelin agonist, relamorelin, in treating constipation in PD, investigators suggested that PD patients may have bowel movements on fewer days than normal, but may experience multiple small, incomplete bowel movements on those days, a phenomenon referred to by the investigators as bowel movements by the installment plan [41]. How common this is or whether it is specific for PD is unknown.
The treatment of slow transit constipation in PD involves a stepwise approach. The first line of treatment is to increase dietary fiber and fluid consumption. For many, perhaps with some additional fiber supplements or bulking agents such as psyllium or methylcellulose and stool softeners such as docusate, that is sufficient [39]. If it is not, the use of laxatives becomes necessary. Chronic daily use of stimulant or irritant laxatives, such as sennosides or bisacodyl, is probably best avoided. Osmotic laxatives, such as polyethylene glycol 3350 and lactulose, which draw fluid into the colon, can be quite effective and seem to be tolerated well even when it is necessary to take them on a daily basis [42]. For individuals in whom these initial treatment approaches have been unsatisfactory, several newer agents with unique mechanisms of action have become available. Lubiprostone, which is a chloride channel activator, was evaluated in one double-blinded, placebo-controlled clinical trial with PD patients and found to be effective [43]. The guanylate cyclase agonist, linaclotide, also can be used, although it has not been specifically studied in PD. The search for more effective agents also continues. Relamorelin, a ghrelin agonist, was evaluated in a multicenter clinical trial but the trial was terminated because of difficulty encountered with enrollment [41]. Novel treatment approaches, such as a vibrating capsule that is swallowed and then activated in the colon to trigger peristalsis, have been successfully utilized in early clinical trials in individuals with chronic constipation but not in the setting of PD [44].
Defecatory dysfunction
Difficulty with the act of defecation itself is the second manifestation of bowel dysfunction in PD. It is characterized by increased straining, incomplete emptying, and painful defecation and is present, at least to some degree, in approximately two-thirds of individuals with PD [10, 45]. For normal defecation to take place, both contraction and relaxation of certain muscles need to occur. The internal and external anal sphincters need to relax, as does the puborectalis muscle, which wraps around the rectum and by tonic contraction produces a kinking in the rectum, similar to a curtain sash, that impedes egress of material and that, with relaxation of the muscle, produces a straightening of the rectum and widening of the anorectal angle that allows easier evacuation [46]. At the same time that these muscles are relaxing, other muscles, such as the abdominal wall muscles, the diaphragm, and even the glottic muscles, need to contract in order to increase intra-abdominal pressure [46]. In PD, this finely choreographed combination of muscle contraction and muscle relaxation becomes uncoordinated and ineffective, resulting in inadequate sphincter relaxation, failure of the anorectal angle to open, and inadequate generation of abdominal pressure with consequent difficulty with defecation, in much the same way that muscular incoordination in the mouth and throat results in dysphagia. These changes have been clearly documented by multiple investigators with the use of both anorectal manometry and defecography [47,48,49].
Treatment of defecatory dysfunction is suboptimal. Pelvic floor exercises are the current standard of treatment. There has been some suggestion that alterations in dopaminergic function may be beneficial. Some patients report that it is easier for them to have a bowel movement when they are “on” with regard to motor function, compared with when they are “off” [unpublished observation]. In keeping with this, both Mathers and Edwards and their colleagues have reported improvement in defecatory function, characterized by improvement in defecography and anorectal manometry, following subcutaneous apomorphine injection in single dose testing in small groups of individuals [47, 49]. Botulinum toxin injections into the external anal sphincter and puborectalis muscles also have been employed successfully but this approach is not widely utilized [50, 51].
Pathophysiology of GI dysfunction in PD
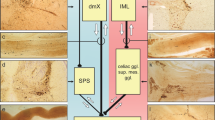
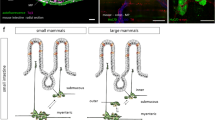
The presence of PD pathology within the GI tract, specifically within the enteric nervous system, was first recognized by Qualman and colleagues in 1984, when they described the presence of Lewy bodies within the esophagus or colon of two PD patients [52]. This was followed by reports by Kupsky and colleagues and Wakabayashi and colleagues of Lewy bodies within the ENS in the colon [53,54,55]. In 1995, Singaram and colleagues took matters one step further when they described both the presence of Lewy bodies and the loss of dopamine neurons primarily within the myenteric plexus in colonic tissue obtained from patients with PD [56]. However, the findings of this study have been called into question by investigators using more modern techniques (see below). Further study was limited by the fact that obtaining myenteric plexus tissue required full-thickness colonic wall biopsy, which was impractical. Thus, investigation languished until it was recognized that Lewy bodies were primarily composed of alpha-synuclein and that alpha-synuclein deposition both within Lewy bodies and as fibrils could be identified by immunohistochemical methods. The current explosion of interest in GI involvement in PD was triggered by the series of reports by Braak and colleagues in which they first proposed that the pathology of PD within the CNS is first apparent in the dorsal motor nucleus of the vagus and spreads from there and then later proposed that pathology may be present even earlier within the ENS and spread via the vagus nerve from the ENS to the CNS [57•]. A number of studies subsequently lent support to the “Braak Hypothesis.”
Abbott and colleagues earlier had reported that individuals with a history of less frequent bowel movements had a higher risk of developing PD [58] and it was subsequently reported that individuals with PD who had experienced constipation prior to the onset of the motor symptoms of their PD had been experiencing the constipation a mean of 18.7 years [59]. More recently, several epidemiological studies, one using a Danish registry and one a Swedish registry, reported that individuals who had undergone full truncal vagotomy for the treatment of ulcer disease were less likely to develop PD than were individuals who had undergone selective or super-selective vagotomy (both of which leave portions of the vagus nerve still intact), which would support the hypothesis that the pathology of PD uses the vagus nerve as a conduit to the brain [60, 61].
The demonstration of alpha-synuclein-positive neurites in submucosal tissue obtained during routine colonoscopy further fueled investigation [62] as did the subsequent report of the presence of submucosal alpha-synuclein-positive neurites in biopsies performed during sigmoidoscopy, which did not require the elaborate preparation necessary with colonoscopy [63]. Shannon and colleagues added to speculation about the possible genesis of PD in the GI tract by reporting the presence of alpha-synuclein deposition in colonic biopsy specimens obtained 2–5 years before the onset of classic PD motor symptoms in patients who were subsequently diagnosed with PD [64]. The presence of alpha-synuclein in individuals diagnosed up to 8 years later with PD was reported by Hilton and colleagues [65].
If PD pathology truly arises within the ENS, presumably triggered by invasion of a pathogen from within the gut lumen, some compromise of the intestinal epithelial barrier must be present. Reports from several investigators have focused on this possibility. Forsyth and colleagues reported that PD subjects exhibit increased colonic permeability and also demonstrated increased intestinal mucosal staining for E.coli, nitro-tyrosine, and alpha-synuclein [66]. Clairembault and colleagues detected alterations in occludin, a tight junction protein involved with control of intestinal barrier permeability, and suggested that these changes could lead to increased permeability [67].
These studies work together to paint a picture that an offending pathogen gains access to enteric neurons via a leaky intestinal epithelial barrier, perhaps setting up a smoldering inflammatory process that results in the formation of abnormal alpha-synuclein that then spreads via the vagus nerve, perhaps in a prion-like fashion, to arrive in the brainstem at the dorsal motor nucleus of the vagus and then to spread from there within the CNs, ultimately producing neuronal damage throughout the brainstem and cerebrum that produces the clinical picture of PD [68,69,70,71]. A variety of animal studies have also been reported that support this formulation, but are beyond the scope of this review.
However, the picture is not quite so pristine [72]. A number of studies also have been published that conflict with the picture portrayed above. Autopsy studies performed on a series of 417 individuals participating in the Brain and Body Donation Program at the Banner Sun Health Institute did not uncover a single case in which Lewy bodies and neurites were present in peripheral autonomic networks but not in the brain [73••]. Furthermore, Annerino and colleagues did not find evidence of any neuronal loss within the ENS of individuals with PD, compared with controls [74]. Objections have also been raised to the Danish vagotomy study [75] and animal studies have been reported that also argue against a gut-to-brain progression of PD pathology [76, 77].
The gut microbiome and the genesis of PD
The most recent explosion of research and writing about the GI system and PD has focused on the gut microbiome and the role it may play in the genesis of PD. Several groups of investigators have reported alterations in the gut microbiome in patients with PD [78,79,80,81]. The reported alterations tend to demonstrate reductions in anti-inflammatory types of bacteria and elevations in pro-inflammatory types in PD patients compared with controls. This, in turn, fuels the speculation that alterations in the gut microbiome may promote an inflammatory environment within the gut and that eventually broaches the epithelial cell barrier and kindles a smoldering inflammatory process within the submucosal and eventually also the myenteric plexus that then is eventually transmitted to the CNS. It is important to note, as pointed out by Quigley, that these studies have generally been relatively small in size and their populations not necessarily representative of the general PD population [82]. In another review, he also points out the possibility that the disease may be driving changes in the microbiome, rather than the microbiome being the driving force [83••].
Animal studies have also provided tantalizing clues regarding the possible role of the gut microbiome in the genesis of neurodegenerative disease. One dramatic example is the report of Sampson and colleagues, in which they utilized an alpha-synuclein transgenic mouse model that develops typical GI and motor features of PD and showed that transgenic mice grown under germ-free conditions exhibited less severe disease than mice grown with normal gut microbiota [84••]. Furthermore, when germ-free transgenic mice received microbiota from humans with PD, they displayed deterioration in motor function [84••].
Conclusion
So, what do we really know about GI dysfunction in PD? It is abundantly clear that GI dysfunction is present in a significant proportion of individuals with PD and that the dysfunction may span the entire GI tract. It is equally clear that there are pathological changes present in both the CNS and the ENS in patients with PD. It is possible, but far from proven, that the pathology of PD may have its genesis within the gut, but it is not so clear whether the GI symptoms of PD are the result of the changes within the ENS or within the CNS—or perhaps both.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Parkinson J. An essay on the shaking palsy. London: Whittingham and Rowland for Sherwood, Neely, and Jones; 1817. A classic treatise that should be read by all neurologists.
Eadie MJ, Tyrer JH. Alimentary disorder in parkinsonism. Australas Ann Med. 1965;14:13–22.
Edwards LL, Pfeiffer RF, Quigley EMM, Hofman R, Baluff M. Gastrointestinal symptoms in Parkinson’s disease. Mov Disord. 1991;6(2):151–6.
Edwards LL, Quigley EM, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease: frequency and pathophysiology. Neurology. 1992;42(4):726–32.
Khoo TK, Yarnall AJ, Duncan GW, Coleman S, O’Brien JT, Brooks DJ, et al. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology. 2013;80(3):276–81.
Fasano A, Visanji NP, Liu LW, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015;14(6):625–39.
Bagheri H, Damase-Michel C, Lapeyre-Mestre M, Cismondo S, O’Connell D, Senard JM, et al. A study of salivary secretion in Parkinson’s disease. Clin Neuropharmacol. 1999;22(4):213–5.
Hyson HC, Johnson AM, Jog MS. Sublingual atropine for sialorrhea secondary to parkinsonism: a pilot study. Mov Disord. 2002;17(6):1318–20.
Narayanaswami P, Geisbush T, Tarulli A, Raynor E, Gautam S, Tarsy D, et al. Drooling in Parkinson’s disease: a randomized controlled trial of incobotulinum toxin A and meta-analysis of Botulinum toxins. Parkinsonism Relat Disord. 2016;30:73–7.
Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Parkinsonism Relat Disord. 2011;17:10–5.
Takizawa C, Gemmell E, Kenworthy J, Speyer R. A systematic review of the prevalence of oropharyngeal dysphagia in stroke, Parkinson’s disease, Alzheimer’s disease, head injury, and pneumonia. Dysphagia. 2016;31(3):434–41.
Pflug C, Bihler M, Emich K, Niessen A, Nienstedt JC, Flügel T, et al. Critical dysphagia is common in Parkinson disease and occurs even in early stages: a prospective cohort study. Dysphagia. 2018;33(1):41–50.
Mu L, Sobotka S, Chen J, Su H, Sanders I, Nvirenda T, et al. Parkinson disease affects peripheral sensory nerves in the pharynx. J Neuropathol Exp Neurol. 2013;72(7):614–23.
Byrne KG, Pfeiffer R, Quigley EM. Gastrointestinal dysfunction in Parkinson’s disease. A report of clinical experience at a single center. J Clin Gastroenterol. 1994;19(1):11–6.
Born LJ, Harned RH, Rikkers LF, Pfeiffer RF, Quigley EMM. Cricopharyngeal dysfunction in Parkinson’s disease: role in dysphagia and response to myotomy. Mov Disord. 1996;11(1):53–8.
Stroudley J, Walsh M. Radiological assessment of dysphagia in Parkinson’s disease. Br J Radiol. 1991;64(766):890–3.
Nagaya M, Kachi T, Yamada T, Igata A. Videofluorographic study of swallowing in Parkinson’s disease. Dysphagia. 1998;13(2):95–100.
Melo A, Monteiro L. Swallowing improvement after levodopa treatment in idiopathic Parkinson’s disease: lack of evidence. Parkinsonism Relat Disord. 2013;19(3):279–81.
Sutton JP. Dysphagia in Parkinson’s disease is responsive to levodopa. Parkinsonism Relat Disord. 2013;19(3):282–4.
Warnecke T, Suttrup I, Schröder JB, Osada N, Oelenberg S, Hamacher C, et al. Levodopa responsiveness of dysphagia in advanced Parkinson’s disease and reliability testing of the FEES-levodopa-test. Parkinsonism Relat Disord. 2016;28:100–6.
Fosso CL, Quigley EMM. A critical review of the current clinical landscape of gastroparesis. Gastroenterol Hepatol (NY). 2018;14(3):140–5.
Goetze O, Wieczorek J, Mueller T, Przuntek H, Schmidt WE, Woitalla D. Impaired gastric emptying of a solid test meal in patients with Parkinson’s disease using 13C-sodium octanoate breath test. Neurosci Lett. 2005;375(3):170–3.
Tanaka Y, Kato T, Nishida H, Yamada M, Koumura A, Sakurai T, et al. Is there a delayed gastric emptying of patients with early-stage, untreated Parkinson’s disease? An analysis using the 13C-acetate breath test. J Neurol. 2011;258(3):421–6.
Heetun ZS, Quigley EM. Gastroparesis and Parkinson’s disease: a systematic review. Parkinsonism Relat Disord. 2012;18(5):433–40.
Su A, Gandhy R, Barlow C, Triadafilopoulos G. Utility of the wireless motility capsule and lactulose breath testing in the evaluation of patients with Parkinson’s disease who present with functional gastrointestinal symptoms. BMJ Open Gastro. 2017;4(1):e000132.
Hasler WL, May KP, Wilson LA, Van Natta M, Parkman HP, Pasricha PJ, et al. Relating gastric scintigraphy and symptoms to motility capsule transit and pressure findings in suspected gastroparesis. Neurogastroenterol Motil. 2018; 30(2). https://doi.org/10.1111/nmo.13196.
Fasano A, Bove F, Gabrielli M, Petracca M, Zocco MA, Ragazzoni E, et al. The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov Disord. 2013;28(9):1241–9.
Sato H, Yamamoto T, Sato M, Furusawa Y, Murata M. Dysphagia causes symptom fluctuations after oral L-DOPA treatment in a patient with Parkinson disease. Case Rep Neurol. 2018;10(1):101–7.
Staisch J, Bakis G, Nutt J. A wrinkle in ON-time – a GI structural abnormality confounding levodopa therapy with Duopa rescue; a case study. Parkinsonism Relat Disord. 2018;50:130–1.
Doi H, Sakakibara R, Sato M, Hirai S, Masaka T, Kishi M, et al. Nizatidine ameliorates gastroparesis in Parkinson’s disease: a pilot study. Mov Disord. 2014;29(4):562–6.
Chedid V, Camilleri M. Relamorelin for the treatment of gastrointestinal motility disorders. Expert Opin Investig Drugs. 2017;26(10):1189–97.
Gil RA, Hwynn N, Fabian T, Joseph S, Fernandez HH. Botulinum toxin type A for the treatment of gastroparesis in Parkinson’s disease patients. Parkinsonism Relat Disord. 2011;17(4):285–7.
Triadafilopoulos G, Gandhy R, Barlow C. Pilot cohort study of endoscopic botulinum neurotoxin injection in Parkinson’s disease. Parkinsonism Relat Disord. 2017;44:33–7.
Shada A, Nielsen A, Marowski S, Helm M, Funk LM, Kastenmeier A, et al. Wisconsin’s Enterra therapy experience: a multi-institutional review of gastric electrical stimulation for medically refractory gastroparesis. Surgery 2018; https://doi.org/10.1016/j.surg.2018.04.043.
Gabrielli M, Bonazzi P, Scarpellini E, Bendia E, Lauritano EC, Fasano A, et al. Prevalence of small intestinal bacterial overgrowth in Parkinson’s disease. Mov Disord. 2011;26(5):889–92.
Tan AH, Mahadeva S, Thalha AM, Gibson PR, Kiew CK, Yeat CM, et al. Small intestinal bacterial overgrowth in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(5):535–40.
Jost WH, Schimrigk K. Constipation in Parkinson’s disease. Klin Wochenschr. 1991;69(20):906–9.
Edwards LL, Quigley EMM, Harned RK, Hofman R, Pfeiffer RF. Characterization of swallowing and defecation in Parkinson’s disease. Am J Gastroenterol. 1994;89(1):15–25.
Ashraf W, Pfeiffer RF, Park F, Lof J, Quigley EMM. Constipation in Parkinson’s disease: objective assessment and response to psyllium. Mov Disord. 1997;12(6):946–51.
Knudsen K, Federova TD, Bekker AC, Iversen P, Østergaard K, Krogh K, et al. Objective colonic dysfunction is far more prevalent than subjective constipation in Parkinson’s disease: a colon transit and volume study. J Parkinsons Dis. 2017;7(2):359–67.
Parkinson Study Group. A randomized trial of relamorelin for constipation in Parkinson’s disease (MOVE-PD): trial results and lessons learned. Parkinsonism Relat Disord. 2017;37:101–5.
Zesiewicz TA, Sullivan KL, Arnulf I, Chaudhuri KR, Morgan JC, Gronseth GS, et al. Practice parameter: treatment of nonmotor symptoms of Parkinson disease. Neurology. 2010;74(11):924–31.
Ondo WG, Kenney C, Sullivan K, Davidson A, Hunter C, Jahan I, et al. Placebo-controlled trial of lubiprostone for constipation associated with Parkinson disease. Neurology. 2012;78(21):1650–4.
Ron Y, Halpern Z, Safadi R, Dickman R, Dekel R, Sperber AD. Safety and efficacy of the vibrating capsule, an innovative non-pharmacological treatment modality for chronic constipation. Neurogastroenterol Motil. 2015;27(1):99–104.
Bassotti G, Maggio D, Battaglia E, Giulietti O, Spinozzi F, Reboldi G, et al. Manometric investigation of anorectal function in early and late stage Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2000;68(6):768–70.
Sun WM, Rao SS. Manometric assessment of anorectal function. Gastroenterol Clin N Am. 2001;30(1):15–32.
Mathers SE, Kempster PA, Law PJ, Frankel JP, Bartram CI, Lees AJ, et al. Anal sphincter dysfunction in Parkinson’s disease. Arch Neurol. 1989;46(10):1061–4.
Edwards LL, Quigley EM, Harned RK, Hofman R, Pfeiffer RF. Defecatory function in Parkinson’s disease: response to apomorphine. Ann Neurol. 1993;33(5):490–3.
Ashraf W, Pfeiffer RF, Quigley EM. Anorectal manometry in the assessment of anorectal function in Parkinson’s disease: a comparison with chronic idiopathic constipation. Mov Disord. 1994;9(6):655–63.
Albanese A, Maria G, Bentivoglio AR, Brisinda G, Cassetta E, Tonali P. Severe constipation in Parkinson’s disease relieved by botulinum toxin. Mov Disord. 1997;12(5):764–6.
Albanese A, Brisinda G, Bentivoglio AR, Maria G. Treatment of outlet obstruction constipation in Parkinson’s disease with botulinum neurotoxin A. Am J Gastroenterol. 2003;98(6):1439–40.
Qualman SJ, Haupt HM, Yang P, Hamilton SR. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson’s disease. Gastroenterology. 1984;87(4):848–56.
Kupsky WJ, Grimes MM, Sweeting J, Bertsch R, Cote LJ. Parkinson’s disease and megacolon: concentric hyaline inclusions (Lewy bodies) in enteric ganglion cells. Neurology. 1987;37(7):1253–5.
Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol. 1988;76(3):217–21.
Wakabayashi K, Takahashi H, Ohama E, Ikuta F. Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol. 1990;79(6):581–3.
Singaram C, Ashraf W, Gaumnitz EA, Torbey C, Sengupta A, Pfeiffer R, et al. Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. Lancet. 1995;346(8979):861–4.
• Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396(1):67–72 Genesis for much of the current discussion of whether PD begins in the gut.
Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57(3):456–62.
Ueki A, Otsuka M. Life style risks of Parkinson’s disease: association between decreased water intake and constipation. J Neurol. 2004;251(Suppl 7):VII18–23.
Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pederson L, Borghammer P, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78(4):522–9.
Liu SY, Chan P, Stoessl AJ. The underlying mechanism of prodromal PD: insights from the parasympathetic nervous system and the olfactory system. Transl Neurodegener. 2017;6:4.
Lebouvier T, Chaumette T, Damier P, Coron E, Touchefeu Y, Vrignaud S, et al. Pathological lesions in colonic biopsies during Parkinson’s disease. Gut. 2008;57(12):1741–3.
Shannon KM, Keshavarzian A, Mutlu E, Dodiya HB, Daian D, Jaglin JA, et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov Disord. 2012;27(6):709–15.
Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord. 2012;27(6):716–9.
Hilton D, Stephens M, Kirk L, Edwards P, Potter R, Zajicek J, et al. Accumulation of α-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 2014;127(2):235–41.
Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, et al. Increased intestinal permeability correlates with sigmoid mucosa alpha synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One. 2011;6(12):e28032.
Clairembault T, Leclair-Visonneau L, Coron E, Bourreille A, Le Dily S, Vavasseur F, et al. Structural alterations of the intestinal epithelial barrier in Parkinson’s disease. Acta Neuropathol Commun. 2015;3:12. https://doi.org/10.1186/s40478-015-0196.
Olanow CW, Prusiner SB. Is Parkinson’s disease a prion disorder? Proc Natl Acad Sci U S A. 2009;106(31):12571–2.
Killinger BA, Labrie V. Vertebrate food products as a potential source of prion-like α-synuclein. NPJ Parkinsons Dis. 2017;3:33.
Borghammer P. How does Parkinson’s disease begin? Perspectives on neuroanatomical pathways, prions, and histology. Mov Disord. 2018;33(1):48–57.
Liddle RA. Parkinson’s disease from the gut. Brain Res. 2018;1693(Pt B):201–6.
Lionnet A, Leclair-Visonneau L, Neunlist M, Murayama S, Takao M, Adler CH, et al. Does Parkinson’s disease start in the gut? Acta Neuropathol. 2018;135(1):1–12.
•• Adler CH, Beach TG. Neuropathological basis of non-motor manifestations of Parkinson’s disease. Mov Disord. 2016;31(8):1114–9 Superb balanced review of the role of ENS involvement in PD.
Annerino DM, Arshad S, Taylor GM, Adler CH, Beach TG, Greene JG. Parkinson’s disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol. 2012;124(5):665–80.
Tysnes OB, Kenborg L, Herlofson K, Steding-Jessen M, Horn A, Olsen JH, et al. Does vagotomy reduce the risk of Parkinson’s disease? Ann Neurol. 2015;78(6):1011–2.
Tasselli M, Chaumette T, Paillusson S, Monnet Y, Lafoux A, Huchet-Caddiou C, et al. Effects of oral administration of rotenone on gastrointestinal functions in mice. Neurogastroenterol Motil. 2013;25(3):e183–93.
Zheng LF, Song J, Fan RF, Chen CL, Ren QZ, Zhang XL, et al. The role of the vagal pathway and gastric dopamine in the gastroparesis of rats after a 6-hydroxydopamine microinjection in the substantia nigra. Acta Physiol (Oxf). 2014;211(2):434–46.
Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, et al. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30(10):1351–60.
Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30(3):350–8.
Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bὕrmann J, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72.
Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, et al. Parkinson’s disease and PD medications have distinct signatures of the gut microbiome. Mov Disord. 2017;32(5):739–49.
Quigley EMM. Microbiota-brain-gut axis and neurodegenerative diseases. Curr Neurol Neurosci Rep. 2017;17(12):94.
• Quigley EMM. Gut microbiome as a clinical tool in gastrointestinal disease management: are we there yet? Nat Rev Gastroenterol Hepatol. 2017;14(5):315–20 Excellent discussion of what is known about the gut microbiome.
•• Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167(6):1469–80 Elegant, innovative, thought-provoking research study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Pfeiffer reports grants from Acorda, personal fees from Lundbeck, personal fees from Cynapsus, personal fees from Clintara, personal fees from Adamas, other from Taylor & Francis (CRC Press), other from Springer (Humana Press), other from Elsevier, and outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Movement Disorders
Rights and permissions
About this article
Cite this article
Pfeiffer, R.F. Gastrointestinal Dysfunction in Parkinson’s Disease. Curr Treat Options Neurol 20, 54 (2018). https://doi.org/10.1007/s11940-018-0539-9
Published:
DOI: https://doi.org/10.1007/s11940-018-0539-9