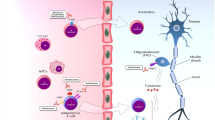
Abstract
Purpose of review
Multiple sclerosis (MS) is an immune-mediated disorder that affects the central nervous system (CNS), often first affecting people in early adulthood. Although most MS patients have a relapsing-remitting course (RRMS) at disease onset, a substantial proportion later develop chronic progression, termed secondary progressive MS (SPMS). Approximately 10% of MS patients experience chronic progression from disease onset, termed primary progressive multiple sclerosis (PPMS). Although several disease-modifying treatment (DMT) options exist for relapsing forms of this disease, DMT options are few for progressive MS (PPMS and SPMS). Herein, we strive to define progressive MS, review major clinical trials aimed at progressive MS, and delineate potential strategies in the management of progressive MS.
Recent findings
In 2017, the first DMT for PPMS, the B lymphocyte-depleting monoclonal antibody, ocrelizumab, came to market. Ocrelizumab reduced 12-week confirmed disability progression (CDP) by 24% versus placebo. Siponimod, a selective sphingosine-1-phosphate receptor modulator, reduced 3-month CDP by 21% versus placebo in SPMS. Ibudilast slowed brain atrophy in PPMS and SPMS patients in a multicenter phase 2b study. Smaller early phase studies of alpha-lipoic acid and simvastatin each found slowing of rate of whole brain atrophy in SPMS patients.
Summary
Reasons now exist for optimism in the search for DMTs for progressive MS. It remains a challenge to identify outcome measures that accurately reflect the underlying pathology in progressive MS, which is less inflammatory and more degenerative than RRMS.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. Neurology. 1996;46:907–11.
Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83:278–86.
Lassmann H, van Horssen J, Mahad DH. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012;8:647–56.
• Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14:183–93. This paper highlights key pathologic mechanisms responsible for neurodegeneration in progressive MS
Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009;8:280–91.
Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–104.
Reynolds R, Roncaroli F, Nicholas R, Radotra B, Gveric D, Howell O. The neuropathological basis of clinical progression in multiple sclerosis. Acta Neuropathol. 2011;122:155–70.
Frischer JM, Weigand SD, Guo Y, Kale N, Parisi JE, Pirko I, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol. 2015;78(5):710–21.
• Lorscheider J, Buzzard K, Jokubaitis V, Spelman T, Havrdova E, Horakova D, et al. Defining secondary progressive multiple sclerosis. Brain. 2016;139:2395–405. Using computer-based analytics, this paper endeavors to create an objective definition of secondary progressive MS using a prospectively-collected database of MS patients
• Ontaneda D, Fox RJ, Chataway J. Clinical trials in progressive multiple sclerosis: lessons learned and future perspectives. Lancet Neurol. 2015;14:208–23. This review of prior clinical trials in progressive MS also critically evaluates clinical trial design in this patient population
Schwid SR, Goodman AD, Apatoff BR, Coyle PK, Jacobs LD, Krupp LB, et al. Are quantitative functional measures more sensitive to worsening MS than traditional measures? Neurology. 2000;55:1901–3.
Lublin FD, Miller DH, Freedman MS, Cree BAC, Wolinsky JS, Weiner H, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387(10023):1075–84.
Alvarez E, Piccio L, Mikesell RJ, Klawiter EC, Parks BJ, Naismith RT, et al. CXCL13 is a biomarker of inflammation in multiple sclerosis, neuromyelitis optica, and other neurological conditions. Mult Scler. 2013;19(9):1204–8.
Komori M, Lin YC, Cortese I, Blake A, Ohayon J, Cherup J, et al. Insufficient disease inhibition by intrathecal rituximab in progressive multiple sclerosis. Ann Clin Transl Neurol. 2016;3(3):166–79.
British And Dutch Multiple Sclerosis Azathioprine Trial Group. Double-masked trial of azathioprine in multiple sclerosis. Lancet. 1988;332(8604):179–83.
The Canadian Cooperative Multiple Sclerosis Study Group. The Canadian cooperative trial of cyclophosphamide and plasma exchange in progressive multiple sclerosis. Lancet. 1991;337(8739):441–6.
Brochet B, Deloire MSA, Perez P, Loock T, Baschet L, Debouverie M, et al. Double-blind controlled randomized trial of cyclophosphamide versus methylprednisolone in secondary progressive multiple sclerosis. PLoS One. 2017;12(1):e0168834.
The Multiple Sclerosis Study Group. Efficacy and toxicity of cyclosporine in chronic progressive multiple sclerosis: a randomized, double-blinded, placebo-controlled clinical trial. Ann Neurol. 1990;27:591–605.
Noseworthy JH, O'Brien P, Erickson BJ, Lee D, Sneve D, Ebers GC, et al. The Mayo Clinic-Canadian cooperative trial of sulfasalazine in active multiple sclerosis. Neurology. 1998;51:1342–52.
Noseworthy JH, Wolinsky JS, Lublin FD, Whitaker JN, Linde A, Gjorstrup P, et al. Linomide in relapsing and secondary progressive MS: part I: trial design and clinical results. Neurology. 2000;54:1726–33.
Goodkin DE, Rudick RA, Medendorp SV, Daughtry MM, Schwetz KM, Fischer J, et al. Low-dose (7.5 mg) oral methotrexate reduces the rate of progression in chronic progressive multiple sclerosis. Ann Neurol. 1995;37:30–40.
Hartung HP, Gonsette R, Konig N, Kwiecinski H, Guseo A, Morrissey SP, et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360:2018–25.
Vermersch P, Benrabah R, Schmidt N, Zéphir H, Clavelou P, Vongsouthi C, et al. Masitinib treatment in patients with progressive multiple sclerosis: a randomized pilot study. BMC Neurol. 2012;12:36.
Rice GPA, Filippi M, Comi G. Cladribine and progressive MS: clinical and MRI outcomes of a multicenter controlled trial. Neurology. 2000;54:1145–55.
Montalban X, et al. Efficacy of cladribine tablets as add-on to IFN-beta therapy in patients with active relapsing MS: final results from the phase II ONWARD study. Neurology. 2016;86(Supplement):P3.029.
European Study Group on Interferon β-1b in Secondary Progressive MS. Placebo-controlled multicenter randomised trial of interferon β-1b in treatment of secondary progressive multiple sclerosis. Lancet. 1998;352(9139):1491–7.
Andersen O, Elovaara I, Färkkilä M, Hansen HJ, Mellgren SI, Myhr KM, et al. Multicentre, randomised, double blind, placebo controlled, phase III study of weekly, low dose, subcutaneous interferon beta-1a in secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 2004;75:706–10.
The North American Study Group on Interferon beta-1b in Secondary Progressive MS. Interferon beta-1b in secondary progressive MS: results from a 3-year controlled study. Neurology. 2004;63:1788–95.
Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon-beta-1a in MS (SPECTRIMS) Study Group. Randomized controlled trial of interferon beta-1a in secondary progressive MS: clinical results. Neurology. 2001;56:1496–504.
Cohen JA, Cutter GR, Fischer JS, Goodman AD, Heidenreich FR, Kooijmans MF, et al. Benefit of interferon beta-1a on MSFC progression in secondary progressive MS. Neurology. 2002;59:679–87.
Leary SM, Miller DH, Stevenson VL, Brex PA, Chard DT, Thompson AJ. Interferon beta-1a in primary progressive MS: an exploratory, randomized, controlled trial. Neurology. 2003;60:44–51.
Montalban X, Sastre-Garriga J, Tintoré M, Brieva L, Aymerich FX, Río J, et al. A single-center, randomized, double-blind, placebo-controlled study of interferon beta-1b on primary progressive and transitional multiple sclerosis. Mult Scler. 2009;15(10):1195–205.
Hawker K, O'Connor P, Freedman MS, Calabresi PA, Antel J, Simon J, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66:460–71.
•• Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209–20. This phase 3 trial of ocrelizumab met its primary endpoint as well as several secondary endpoints, leading to its approval for PPMS
Hartung HP, et al. ASCEND phase 3 trial open-label extension study results: natalizumab may delay disability progression in secondary progressive multiple sclerosis (SPMS). Neurology. 2017;88(Supplement):P5.330.
Wolinsky JS, Narayana PA, O'Connor P, Coyle PK, Ford C, Johnson K, et al. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann Neurol. 2007;61:14–24.
•• Chaudhry BZ, Cohen JA, Conway DS. Sphingosine 1-phosphate receptor modulators for the treatment of multiple sclerosis. Neurotherapeutics. 2017;14:859–73. https://doi.org/10.1007/s13311-017-0565-4. This review of clinical trials of S1P receptor modulators reviews results of the phase 3 EXPAND trial of siponimod in SPMS.
Hommes OR, Sørensen PS, Fazekas F, Enriquez MM, Koelmel HW, Fernandez O, et al. Intravenous immunoglobulin in secondary progressive multiple sclerosis: randomised placebo-controlled trial. Lancet. 2004;364:1149–56.
Pöhlau D, Przuntek H, Sailer M, Bethke F, Koehler J, König N, et al. Intravenous immunoglobulin in primary and secondary chronic progressive multiple sclerosis: a randomized placebo controlled multicenter study. Mult Scler. 2007;13:1107–17.
•• Tourbah A, Lebrun-Frenay C, Edan G, Clanet M, Papeix C, Vukusic S, et al. MD1003 (high-dose biotin) for the treatment of progressive multiple sclerosis: a randomised, double-blind, placebo-controlled study. Mult Scler. 2016;22(13):1719–31. The results of this phase 3 trial of high-dose biotin in progressive MS patients demonstrated significant disability reversal in a small proportion of the treatment group
•• Spain R, Powers K, Murchison C, Heriza E, Winges K, Yadav V, et al. Lipoic acid in secondary progressive MS. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e374. This phase 2 trial of alpha-lipoic acid in SPMS demonstrated 68% reduction in annualized percent change in brain volume versus placebo
•• Chataway J, Schuerer N, Alsanousi A, Chan D, MacManus D, Hunter K, et al. Effect of high-dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS-STAT): a randomised, placebo-controlled, phase 2 trial. Lancet. 2014;383:2213–21. This phase 2 trial of simvastatin in SPMS showed 43% reduction in annualized percent change in brain volume versus placebo
Kapoor R, Furby J, Hayton T, Smith KJ, Altmann DR, Brenner R, et al. Lamotrigine for neuroprotection in secondary progressive multiple sclerosis: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Neurol. 2010;9:681–8.
Freedman MS, Bar-Or A, Oger J, Traboulsee A, Patry D, Young C, et al. A phase III study evaluating the efficacy and safety of MBP8298 in secondary progressive MS. Neurology. 2011;77:1551–60.
Zajicek J, Ball S, Wright D, Vickery J, Nunn A, Miller D, et al. Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): a randomised, placebo-controlled trial. Lancet Neurol. 2013;12:857–65.
Schreiber K, Magyari M, Sellebjerg F, Iversen P, Garde E, Madsen CG, et al. High-dose erythropoietin in patients with progressive multiple sclerosis: a randomized, placebo-controlled, phase 2 trial. Mult Scler. 2017;23(5):675–85.
McCroskery P, et al. Safety and tolerability of opicinumab in relapsing multiple sclerosis: the phase 2b SYNERGY trial. Neurology. 2017;88(Supplement):P5.369.
Giovannoni G, Cutter G, Sormani MP, Belachew S, Hyde R, Koendgen H, et al. Is multiple sclerosis a length-dependent central axonopathy? The case for therapeutic lag and the asynchronous progressive MS hypotheses. Mult Scler Relat Disord. 2017;12:70–8.
Lugaresi A, Caporale C, Farina D, Marzoli F, Bonanni L, Muraro PA, et al. Low-dose oral methotrexate treatment in chronic progressive multiple sclerosis. Neurol Sci. 2001;22(2):209–10.
Martinelli-Boneschi F, et al. Mitoxantrone for multiple sclerosis. Cochrane Database Syst Rev. 2013;5:CD002127.
Li DKB, Zhao GJ, Paty DW, University of British Columbia MS/MRI Analysis Research Group. The SPECTRIMS Study Group. Randomized controlled trial of interferon beta-1a in secondary progressive MS, MRI results. Neurology. 2001;56:1505–13.
Kappos L, Weinshenker B, Pozzilli C, Thompson AJ, Dahlke F, Beckmann K, et al. Interferon beta-1b in secondary progressive MS: a combined analysis of the two trials. Neurology. 2004;63:1779–87.
La Mantia L, et al. Interferon β for secondary progressive multiple sclerosis: a systematic review. J Neurol Neurosurg Psychiatry. 2013;84:420–6.
Sorensen PS, Blinkenberg M. The potential role for ocrelizumab in the treatment of multiple sclerosis:current evidence and future prospects. Ther Adv Neurol Disord. 2016;9(1):44–52.
Helliwell CL, Coles AJ. Monoclonal antibodies in multiple sclerosis treatment: current and future steps. Ther Adv Neurol Disord. 2009;2(4):195–203.
Paolillo A, Coles AJ, Molyneux PD, Gawne-Cain M, MacManus D, Barker GJ, et al. Quantitative MRI in patients with secondary progressive MS treated with monoclonal antibody Campath 1H. Neurology. 1999;53:751–7.
Coles AJ, Cox A, Page E, Jones J, Trip SA, Deans J, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol. 2006;253:98–108.
Coles AJ. Alemtuzumab therapy for multiple sclerosis. Neurotherapeutics. 2013;10:29–33.
Christensen JR, et al. Natalizumab in progressive MS; results of an open-label, phase 2A, proof-of-concept trial. Neurology. 2014;82:1499–507.
Kappos L, Li DKB, Stüve O, Hartung HP, Freedman MS, Hemmer B, et al. Safety and efficacy of siponimod (BAF312) in patients with relapsing-remitting multiple sclerosis. JAMA Neurol. 2016;73(9):1089–98.
Zhornitsky S, Wee Yong V, Koch MW, Mackie A, Potvin S, Patten SB, et al. Quetiapine fumarate for the treatment of multiple sclerosis: focus on myelin repair. CNS Neurosci Ther. 2013;19:737–44.
Sedel F, Papeix C, Bellanger A, Touitou V, Lebrun-Frenay C, Galanaud D, et al. High doses of biotin in chronic progressive multiple sclerosis: a pilot study. Mult Scler Relat Disord. 2015;4:159–69.
Sedel F, Bernard D, Mock DM, Tourbah A. Targeting demyelination and virtual hypoxia with high-dose biotin as a treatment for progressive multiple sclerosis. Neuropharmacology. 2016;110:644–53.
Barkhof F, Hulst HE, Drulovic J, Uitdehaag BMJ, Matsuda K, Landin R, et al. Ibudilast in relapsing-remitting multiple sclerosis: a neuroprotectant? Neurology. 2010;74:1033–40.
Derfuss T, Curtin F, Guebelin C, Bridel C, Rasenack M, Matthey A, et al. A phase IIa randomised clinical study of GNbAC1, a humanised monoclonal antibody against the envelope protein of multiple sclerosis-associated endogenous retrovirus in multiple sclerosis patients. Mult Scler. 2015;21(7):885–93.
Zhornitsky S, Yong VW, Weiss S, Metz LM. Prolactin in multiple sclerosis. Mult Scler. 2012;19(1):15–23.
Kremer D, Küry P, Dutta R. Promoting remyelination in multiple sclerosis: current drugs and future prospects. Mult Scler. 2015;21(5):541–9.
Mi S, Pepinsky RB, Cadavid D. Blocking LINGO-1 as a therapy to promote CNS repair: from concept to the clinic. CNS Drugs. 2013;27:493–503.
Cadavid D, Balcer L, Galetta S, Aktas O, Ziemssen T, Vanopdenbosch L, et al. Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:189–99.
Desmukh VA, et al. A regenerative approach to the treatment of multiple sclerosis. Nature. 2013;502:327–32.
Mei F, Fancy SPJ, Shen YAA, Niu J, Zhao C, Presley B, et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat Med. 2014;20(8):954–60.
Green AJ, Gelfand JM, Cree BA, Bevan C, Boscardin WJ, Mei F, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet. 2017;390:2481–9. https://doi.org/10.1016/S0140-6736(17)32346-2.
Warrington AE, Bieber AJ, Ciric B, Pease LR, van Keulen V, Rodriguez M. A recombinant human IgM promotes myelin repair after a single, very low dose. J Neurosci Res. 2007;85(5):967–76.
Demicheva E, Cui YF, Bardwell P, Barghorn S, Kron M, Meyer AH, et al. Targeting repulsive guidance molecule a to promote regeneration and neuroprotection in multiple sclerosis. Cell Rep. 2015;10(11):1887–98.
O’Leary C, et al. RGMa regulates cortical interneuron migration and differentiation. PLoS One. 2013;8(11):e81711.
Volpe G, Bernstock JD, Peruzzotti-Jametti L, Pluchino S. Modulation of host immune responses following non-hematopoietic stem cell transplantation: translational implications in progressive multiple sclerosis. J Neuroimmunol. 2016; https://doi.org/10.1016/j.jneuroim.2016.12.005.
Rice CM, Mallam EA, Whone AL, Walsh P, Brooks DJ, Kane N, et al. Safety and feasibility of autologous bone marrow cellular therapy in relapsing-progressive multiple sclerosis. Clin Pharmacol Ther. 2010;87(6):679–85.
Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11(2):150–6.
Harris VK, Vyshkina T, Sadiq SA. Clinical safety of intrathecal administration of mesenchymal stromal cell-derived neural progenitors in multiple sclerosis. Cytotherapy. 2016;18:1476–82.
Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12:252–64.
Acknowledgements
Anne Haney Cross was supported in part by the Manny and Rosalyn Rosenthal–Dr. John L. Trotter MS Center Chair in Neuroimmunology of the Barnes-Jewish Hospital Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
John Robert Ciotti declares no conflict of interest.
Anne Haney Cross reports personal fees from Abbvie, Biogen, Bayer Healthcare, EMD Serono, Genzyme (Sanofi), Genentech (Roche), Novartis, and Teva Neuroscience.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Multiple Sclerosis and Related Disorders
Rights and permissions
About this article
Cite this article
Ciotti, J.R., Cross, A.H. Disease-Modifying Treatment in Progressive Multiple Sclerosis. Curr Treat Options Neurol 20, 12 (2018). https://doi.org/10.1007/s11940-018-0496-3
Published:
DOI: https://doi.org/10.1007/s11940-018-0496-3